Organic Chemistry – Some Basic Principles and Techniques: Class 11 Chemistry NCERT Chapter 12

Key Features of NCERT Material for Class 11 Chemistry Chapter 12 – Organic Chemistry – Some Basic Principles and Techniques
In the last chapter 11, you studied about p-block and its properties. In this chapter: Organic Chemistry – Some Basic Principles and Techniques, you will learn about many things about Organic chemistry.
Organic compounds comprise about 90% of everything being equal. That is a considerable amount, isn’t it? So as to study countless such compounds, it is necessary to classify them. Tell us more about the Classification of Organic Compounds as well as the overall categories into which organic compounds are partitioned.
Quick revision notes
- Organic Chemistry
Organic chemistry deals with the study of hydrocarbons and their derivatives.
The Shapes of Carbon Compounds:
Inorganic or carbon compounds, s, and p orbitals are engaged with hybridization. This leads to three types of hybridization, which are sp3(in alkanes) – Tetrahedral fit as a fiddle sp2(in alkenes) – Planar structure sp(in alkynes) – Straight molecule.
Functional Group: A single atom or group of atoms that participate in a specific way to determine the organic compound’s chemical properties. The examples are hydroxyl group (— Goodness), aldehyde group (— CHO), and carboxylic acid group (— COOH), and so on.
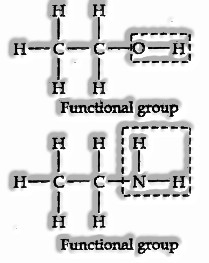
Homologous Series
A homologous series might be characterized as a group of organic compounds having the same functional group, similar chemical properties, and the successive members contrast from one another in the molecular formula by — CH2 units.
The same general molecular formula can represent the members of a homologous series.
- Nomenclature of Organic Compounds
Common Name (Basic system): Before the IUPAC system of nomenclature, organic compounds were named after the sources of the cause; for instance, urea was so named because it was acquired from the urine of mammals. Formic acid was named when it was disengaged from red ants called Formica.
*IUPAC (International Union of Pure and Applied Chemistry) System
As indicated by the IUPAC system, the name of an organic compound comprises three parts: the word root, suffix, and prefix.
(I) Word root indicates the number of carbon atoms present in the principal chain, which is the longest possible chain of carbon atoms.
(ii) Suffix: It is primarily of two types: primary and secondary suffix.
(a) Primary Suffix: It shows the type of bond between the atoms of carbon.
(b) Secondary Suffix: It is used to represent the functional group.
(iii) Prefix: Prefix is a piece of IUPAC name that appears before the word root. A prefix is of two types:
(a) Primary prefix: For instance, primary prefix cyclo is used to separate cyclic compounds.
b) Secondary prefix: Few functional groups are considered as substituents and denoted by secondary prefixes.
For instance:
Substituted Group Secondary prefix.
— F Flupro
— Cl Chloro
— Br Bromo
— NO Nitroso
— NO2 Nitro
— CH3 Methyl
— OCH3 Methoxy
-
Isomerism
When at least two compounds possess the same molecular formula yet extraordinary structural formula and diverse properties(both physical and chemical), the phenomenon is called isomerism, and Such compounds are called isomers.
It is mainly of two types:
(1) Structural Isomerism
(2) Stereoisomers
(1) Structural Isomerism: Structural isomerism is when the compounds have the same molecular formula; however, extraordinary structural formulae contrasting in the course of action of atoms.
(2) Stereoisomerism: When isomerism is caused by the various arrangements of atoms or groups in space, the phenomenon is called stereoisomerism. The stereoisomers have the same structural formula however vary in the game plan of atoms in space. Stereoisomerism is of two types:
(I) Geometrical or Cis-Trans Isomerism
(ii) Optical Isomerism
- Crucial Concepts:
covalent bond fission: A covalent bond experiences Fission in two ways:
(I) By Homolytic Fission or Homolysis
(ii) By Heterolytic Fission or Heterolysis
Homolytic fission: In this process, every one of the atoms acquires one of the bonding electrons.

Heterolytic Fission: In this process, one atom takes up both of the bonding electrons when the bond is broken.
In the event that B is more electronegative than A, which along these lines acquires both the bonding electrons and gets negatively charged. The final product of heterolytic Fission is ions ultimately.
Reaction Intermediates: Both the Heterolytic and homolytic bond fission results in the development of short fragments called reaction intermediates. Among the significant reactions, intermediates are carbonium ions, carbanions, carbon-free radicals, and carbenes. Carbonium Ions (carbocations): Organic ions that contain a positively charged carbon atom are called carbonium ions or carbocations. They are framed by heterolytic bond fission.
![]()
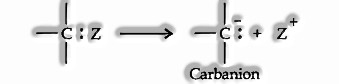
Where Z is more electronegative than carbon.
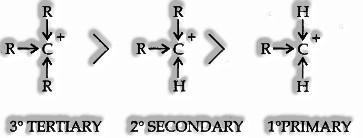
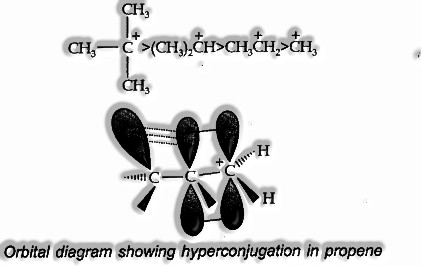
Tertiary carbonium particle is more stable than a secondary, which like this, is more durable than a primary because of the +1 effect associated with the alkyl group.

Carbanion: Organic particles which contain a negatively charged carbon atom are called carbanions.
Where carbon is more electronegative than Z, a primary carbanion is more stable than a secondary, which like this, is more durable than a tertiary because of the +1 effect associated.
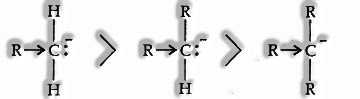
Electrophile: An electron insufficient positively charged or neutral species are known as Electrophiles, e.g.,
He–, H20+, CH3, NH4+, AlCl3.
Nucleophile: They are negatively charged or neutral species having lone pair of electrons, e.g., (HO–), Cyanide (C = N), H20: R3N, R2NH, and so on.
Electron Displacement Effects in Covalent Bonds: This occurs because of the presence of an atom or group of various electronegativity or affected by some outside connecting group.
These lead to various effects which are as follows:
(I) Inductive effect (ii) Elecromeric effect
(iii) Resonance or Mesomeric effect (iv) Hyperconjugation effect.
Inductive Effect: It involves c electron. The electrons which structure a covalent bond are seldom shared similarities between the two atoms. Because of various electronegative electrons are displaced towards the more electronegative atom. This introduces a specific level of polarity.
The more electronegative atom takes up a small negative charge (δ–). The less electronegative atom acquires a slightly positive charge (δ+).
Consider the carbon-chlorine bond.
As Chlorine is more electronegative, it will turn out to be negatively charged concerning the carbon atom.
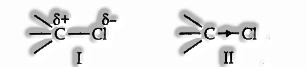
Structure I-indicates the relative charges on the two atoms.
Structure II-indicates the electrons are removed.
Atoms or groups which lose electrons to a carbon atom are said to have a +1 effect. Those atoms or groups which draw electrons from a carbon atom are said to have a – I Effect.
Some basic atoms or groups which cause +I or – I effects are shown underneath:
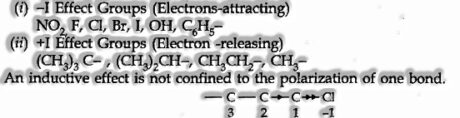
The chlorine atom has higher inductive effect than C3 and C2.
Resonance Structure: various organic compounds cannot be precisely represented by one structure.
For instance, benzene is customarily represented as

Carbon-carbon double bond length = 1.34 A
Carbon-carbon single bond length = 1.54A. However, it has been resolved tentatively that all carbon-carbon bonds in benzene are indistinguishable and have an equal bond length (1.39A).
Hence, the structure of benzene cannot be represented by a single structure. It tends to be represented similarly well by ‘he vigorously similar structures I and II. The two structures are called resonance structures.
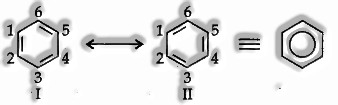
The original structure of benzene is a resonance hybrid of structures I and II.
Another case of resonance is given by nitromethane (CH3N02), which can be represented by two Lewis structures.
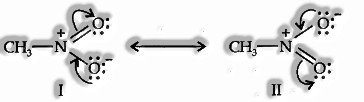
The genuine structure of benzene is a resonance hybrid of structures I and II.
Another case of resonance is given by nitromethane (CH3N02), which can be represented by two Lewis structures.
The genuine structure of benzene is a resonance hybrid of structures I and II.
Another case of resonance is given by nitromethane (CH3N02), which can be represented by two Lewis structures.
The genuine structure of nitromethane is a resonance hybrid of the two canonical forms I and II. Resonance vitality: The distinction in the vitality between the most stable contributing structure for a compound and its resonance hybrid is called resonance vitality or resonance stabilization vitality.
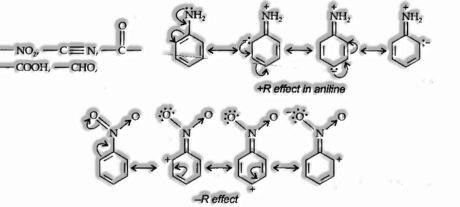
Resonance Effect: The polarity created in the molecule by the association of two π-bonds or between a π-bond and a lone pair of electrons present on a nearby atom. There are two kinds of resonance or mesomeric effects named R or M effect, respectively.
Positive Resonance Effect (+R effect): The atoms which lose electrons to a carbon atom are said to have a +M effect or +R effect. For instance:
— Cl, — Br, — I, — NH2, — NR2, — Gracious, — OCH3
Negative Resonance Effect (- R effect): Those atoms or groups which draw electrons from a carbon atom are said to have a – M effect or – R effect.
For instance:
Electromeric Effect (E Effect):
The electromeric effect refers to the polarity created in numerous bonded compounds when it is assaulted by a reagent when a double or a triple bond is exposed to an assault by an electrophile E+ (a reagent) the two π electrons which from the π bond are transferred to each other. The electromeric effect is shown below:

The curved arrow shows the displacement of the electron pair. The atom A has lost its share in the electron pair, and B has gained this share. Therefore A acquires a positive charge and B a negative charge.
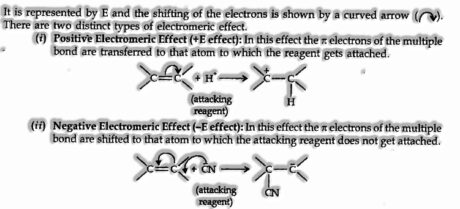
The bent arrow signifies the shifting of the electron pair. The atom B has gained this share, and atom A lost its pair. Therefore A gets a positive charge, and B will automatically have a negative charge on it.
Hyperconjugation: When the alkyl is appended to an unsaturated system such as — CH=CH2 group, the request for the inductive effect gets reversed. The hyperconjugation effect can clarify the conduct.
Such structures are shown up by shifting the bonding electrons from an adjoining C — H bond to the electron lacking carbon. In this manner, the positive charge initially on carbon is dispersed to the hydrogen.
This electron release method assumes no bond character in the nearby C—H bond is called No-Bond Resonance or Hyperconjugation.
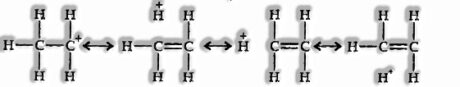
-
Orbital Idea of Hyperconjugation
It involves delocalization of o electrons of the C—H bond of an alkyl group connected legitimately to an atom of unsaturated system or an atom having unshared p-orbital.
Let us consider CH3CH2 (ethyl cation), which has positively charged carbon atom having a vacant p-orbital. Anyone of the C—H bonds of the methyl group can adjust in the plane of this unfilled p-orbital. Electron constituting the C—H bond in-plane with this p-orbital would then be able to be delocalized into the vacant p-orbital, as in Fig.
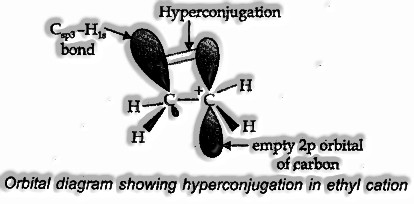
When all is said is done, more prominent the quantity of alkyl groups connected to a positively charged carbon atom, the more prominent is the hyperconjugation.
- Subjective Analysis of Organic Compounds
Discovery of Carbon and hydrogen: Put Copper Oxide Test:
The organic substance is blended personally with around three times its weight of dry copper oxide. The blend is then positioned in a hard glass test-tube fitted with a twisted conveyance tube. The opposite finish of which is dunking into lime water in another test tube.
The blend is warmed strongly, and the accompanying reactions happen.
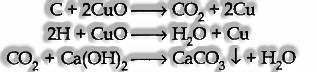
Thus if carbon is present, it is oxidized to carbon dioxide, which turns lime water smooth. In the event that hydrogen is also present, it will be oxidized to water droplets on the test tube’s cooler mass.
Recognition of different elements
Lassaigne’s test: Nitrogen, sulfur, halogens, and phosphorus present in an organic compound are distinguished by ‘Lassaigne’s test.’ Covalent compounds are changed over into ionic structure by fusing the compound with sodium metal. Following reaction occurs:
Thus if carbon is present, it is oxidized to carbon dioxide, which makes lime water milky. If hydrogen is mixed, it will be oxidized to water droplets on the test tube’s cooler wall.

Test for Nitrogen:
(I) The substance is warmed actively with sodium metal.
Na + C + N — – > NaCN
(ii) The water concentrate of the fused mass is overflowed with ferrous sulphate solution
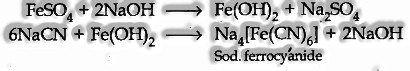
(iii) The cooled solution is then included somewhat ferric chloride solution and an excess of concentrated hydrochloric acid.
![]()
The formation of Prussian blue or green coloration predicts the nitrogen in it.
Test for sulphur:
Sodium Test: Sulphur, in the given organic compound, when fused with sodium reacts to form sodium sulphide
![]()
Thus the sodium remove got from the fused mass is tested as:
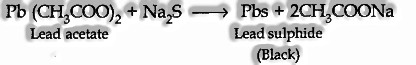
(I) Include freshly arranged sodium nitroprusside solution. A profound violet coloration indicates sulfur.
(ii) Acidify the bit of the concentrate with acetic acid and then include lead acetate solution. A dark precipitate of lead sulfide confirms the presence of sulphur.
In the event that both nitrogen and sulfur are present in an organic compound, sodium thiocyanate is framed. It gives dark red shading and no Prussian blue as there are no free cyanide ions present.
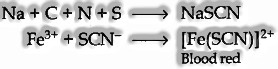
Test for Halogens
Sodium Test: On fusion with sodium, the halogens present in the organic compound are changed over to sodium halides.
Cl + Na — – > NaCl
Br + Na — > NaBr
I + Na — >NaI
Acidify a part of ‘sodium extricate’ with weaken nitric acid and add to its silver nitrate solution—white ppt. Soluble in ammonia indicates Chlorine.
Yellow ppt. Sparingly soluble in ammonia indicates Bromine.
Yellow ppt. Insoluble in ammonia indicates Iodine.
When nitrogen or sulfur is also present in the compound, the sodium extricates before testing for halogens are overflowed with strong nitric acid to decompose the cyanide and the sulphite shaped during the sodium fusion. If not expelled, these radicals will frame a white and dark precipitate respectively on the expansion of silver nitrate.
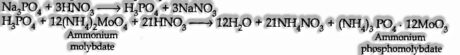
Test for phosphorus
The compound is warmed with an oxidizing operator (sodium peroxide). The present phosphorus in the compound is oxidized to phosphate.
The solution is overflowed with HN03 and treated with ammonium molybdate. A yellow-hued ppt. Indicates the presence of phosphorus.
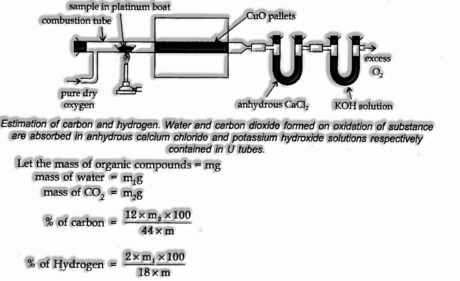
Estimation of Carbon and Hydrogen
![]()
The presence of both carbon and hydrogen are estimated together in one activity. A known load of an organic compound is scorched in excess oxygen and copper (II) oxide. Carbon and hydrogen are oxidized to carbon dioxide and water, respectively.
The heaviness of carbon dioxide and water thus shaped are resolved, and the amounts of carbon and hydrogen in the first substance are determined.
The weight of carbon dioxide and water ultimately formed is determined, and the amounts of carbon and hydrogen in the original substance calculated.
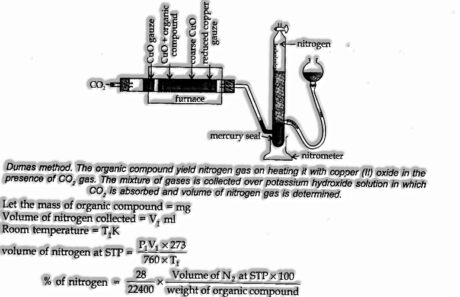
Estimation of Nitrogen
(I) Dumas method: This method is based on the actuality that the nitrogenous compounds when warmed with copper oxide in an atmosphere of carbon dioxide yield free nitrogen,
The traces of oxides of nitrogen, which might be shaped in some cases, are decreased to necessary nitrogen by passing over a warmed copper spiral.
Apparatus showed below:
(ii) Kjeldahl’s Methods: Kjeldahl’s method has relied on how an organic compound containing nitrogen is warmed with con. H2S04 the nitrogen in it is changed over to ammonium sulfate. The resultant fluid is then treated with an excess of soluble base and the freed ammonia gas absorbed over standard acid. The measure of ammonia is dictated by finding the measure of acid neutralized by back filtration with some std. Soluble base.

Every single organic compound containing carbon and hydrogen as essential constituents.
- In a homologous series, two successive members vary in their molecular formula by – CH2 unit.
- Aliphatic Compounds are open-chain compounds contain straight or spread chain of carbon atoms.
- Alicyclic Compounds: Compounds containing a closed ring of carbon.
- Aromatic Compounds: Benzene and its derivatives are known as aromatic compounds.
- Functional group: A functional group is formed when an atom or group of atoms bonded together extraordinarily and determines the compounds’ physical and chemical properties.
- Homolytic Bond Fission: It leads to the development of free radicals.

Crystallization is used to refine organic solids by dissolving them in suitable solvents.
- Simple distillation is used to refine liquids with non-unpredictable impurities.
- Steam distillation is used to improve organic compounds that give sufficient vapors at the bubbling of water and are insoluble in water.
- Chromatography is used to refine and separate the constituents from a sample.
- Lassaigne’s test is used to identify carbon, nitrogen, sulfur, and halogen in a compound.
- Dumas or Kjeldahl’s method: Nitrogen can be estimated by this method.
- Halogens: Halogens are assessed via the Carius method.
- Sulfur and phosphorous: Sulfur and phosphorous are estimated by oxidizing them to sulphuric and phosphoric acid.
- Oxygen: The level of oxygen is usually controlled by the distinction between the absolute rate (100) and the sum of the percentages of every single other component present.


