Haloalkanes And Haloarenes: Class 12 Chemistry NCERT Chapter 10

Key Features of NCERT Material for Class 12 Chemistry Chapter 10 – Haloalkanes And Haloarenes
In the previous chapter 9: Coordination Compounds, you learned about the gander at the significance and uses of coordination mixes. We will take a gander at the functional utilizations of these significant mixes. In this chapter: Haloalkanes And Haloarenes, you will learn about that why haloalkanes and haloarenes endure in the earth? Haloarenes were utilized as herbicides during Vietnam War for defoliating the wildernesses and making it simpler to battle in the war. These haloarenes can’t be deteriorated and separated by microorganisms like microbes. Because of which it is as yet flawless in the dirts of the wildernesses right up ’til today.
Haloalkanes and Haloarenes
At the point when a hydrogen particle in an aliphatic or fragrant hydrocarbon is supplanted by halogen iotas then the mixes are named as haloalkanes and haloarenes. On the off chance that a hydrogen iota is supplanted from an aliphatic hydrocarbon by a halogen molecule the subsequent compound framed is called as haloalkane. It is otherwise called alkyl halide and halogenalkane.
Be that as it may, if a hydrogen molecule is supplanted from a fragrant hydrocarbon by a halogen particle the subsequent compound framed is known as haloarene. It is otherwise called aryl halide or halogenoarene. In a haloalkene (R – X), X speaks to halogen gathering. It is appended to a sp3 hybridized molecule of an alkyl gathering though in haloarene (Ar – X) the halogen is connected to a sp2 hybridized particle of an aryl gathering.
Quick revision notes
- Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive charge and X having a partial negative charge.
- Preparation of haloalkanes:

- a) By elecrophilic substitution reaction:


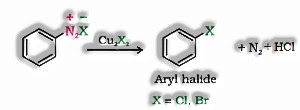
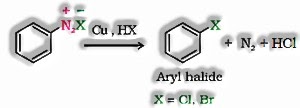
- b) Sandmeyer’s reaction:


- c) Gattermann reaction:

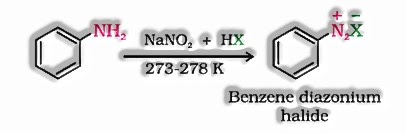
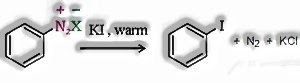
- d) From Diazonium Chloride:

e). Balz – Schiemann reaction: 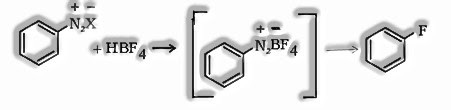
- Physical properties of haloalkanes:
- a) Solubility
- In spite of the fact that haloalkanes are polar in nature, yet they are for all intents and purposes marginally solvent in water.
- All together for a haloalkane to disintegrate in water, vitality is required to defeat the attractions between the haloalkane atoms and break the hydrogen securities between water particles.
- Anyway Haloalkanes can’t shape hydrogen bonds with water and consequently, less vitality is delivered when new attractions are set up between the haloalkane and the water particles in light of the fact that these are not as solid as the first hydrogen bonds in water atoms.
- Thus, dissolvability of haloalkanes in water is low.
- b) Density
- Straightforward fluoro and chloroalkanes are lighter than water while bromides and polychlorodevrivatives are heavier than water.
- With the expansion in number of carbon iotas, the densities continue expanding. With the expansion in number of halogen particles, the densities continue expanding. The densities increment in the request: Fluoride < chloride < bromide < iodide
- The thickness additionally increments with expanding number and nuclear mass of the halogen.
- c) Boiling Points
- Atoms of natural halogen mixes are commonly polar.
- Because of the extremity just as higher atomic mass when contrasted with the parent hydrocarbon, the intermolecular powers of fascination (dipole – dipole and van der Waals) between the particles are more grounded in halogen subordinates of alkanes.
- Accordingly softening and breaking points of chlorides, bromides and iodides are significantly higher than those of the parent hydrocarbon of tantamount atomic mass.
- For a similar alkyl bunch the breaking points of alkyl chlorides, bromides and iodides follow the request RI >RBr>RCl> RF where R is an alkyl gathering. This is on the grounds that with the expansion in the size of the halogen, the size of van der Waals power increment.
- As a rule, the breaking points of chloro, bromo and iodo mixes increment with increment in the quantity of halogen particles.
- For a similar halogen molecule, the breaking points of haloalkanes increment with increment in the size of alkyl gatherings.
- For isomeric alkyl halides, the breaking points decline with expanding. This is on the grounds that spreading of the chain makes the particle more conservative and, accordingly, decline the surface zone. Because of abatement in surface territory, the greatness of van der Waals powers of fascination diminishes and subsequently, the breaking points of the expanded chain compound is not exactly those of the straight chain mixes.
- Physical Properties of Haloarenes:
- These are commonly dreary fluids or glasslike solids.
- These are heavier than water.
- Softening and breaking points of haloarenes
- Softening and breaking points of haloarenes are almost equivalent to those of alkyl halides containing a similar number of carbon particles.
- The breaking points of monohalogen subsidiaries of benzene are in the request: iodo>bromo>chloro>fluoro
iii. For a similar halogen iota, the liquefying and breaking points increment as the size of the aryl bunch increments.
- The softening purpose of para isomer is very higher than that of ortho or meta isomers. This is because of the quick that is has even structure and accordingly, its particles can without much of a stretch pack freely in the precious stone grid. Subsequently intermolecular powers of fascination are more grounded and in this manner, more noteworthy vitality is required to break its grid and it softens at higher temperature.
- Chemical properties of haloalkanes:
Nucleophilic substitution reaction:

Mechanism of Nucleophilic Substitution Reaction:
SN1 Mechanism
- First order reaction.
- Rate = k [RX] [Nu]
- Racemic mixture
- One step reaction
- Order: CH3X < 10< 20< 30
SN2 Mechanism
-
- Second order reaction
- Rate = k [RX]
- Inversion of configuration
- Two step reaction
- Order: CH3X > 10> 20> 30


- Disposal response: Dehydrohalogentaion(– end): When a haloalkane with β-hydrogen molecule is warmed with alcoholic arrangement of potassium hydroxide, there is end of hydrogen iota from β-carbon and a halogen particle from the α-carbon molecule. Therefore, an alkene is shaped as an item. Zaitsev rule (additionally articulated as Saytzeff) is followed.It states that “In dehydrohalogenation responses, the favored item is that alkene which has the more noteworthy number of alkyl bunches appended to the doubly reinforced carbon molecules.”
- Response with metals:
- a) Reaction with Magnesium

- b) Wurtz reaction

- Chemical properties of haloarenes:
- a) Dow’s Process

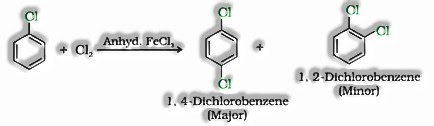
- b) With halogens

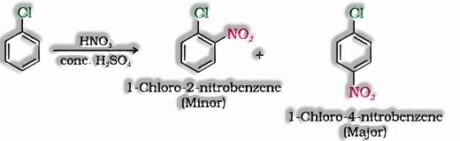
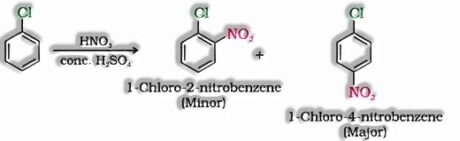
- c) With conc. nitric and sulphuric acid

- d) On heating with conc. sulphuric acid

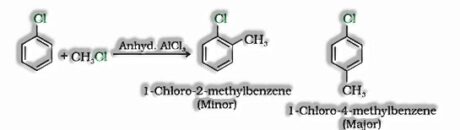
- e) With methyl chloride

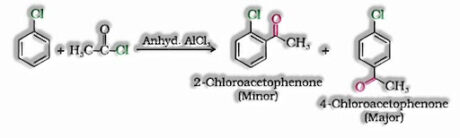
- f) With acetyl chloride



Question
Q: Identify 1°, 2° and 3° haloalkanes from the structures given underneath.
haloalkanes
Arrangement: 1)- Primary (1° haloalkane), 2)- Tertiary (3° haloalkane), 3)- Secondary (2° haloalkane)