Electrochemistry: Class 12 Chemistry NCERT Chapter 3
Characteristics of NCERT Class 12 Chemistry Chapter 3 – Electrochemistry
In the last chapter 2, you learned about solutions. In this chapter: Electrochemistry you will learn about Electrochemistry is a part of chemistry that manages the investigation of electricity production from energy delivered during unconstrained chemical reactions and the utilization of electrical energy to bring about non-unconstrained chemical transformations. We should discover more.
Significance of Electrochemistry
- Electroplating.
- Electroplating Batteries and cells utilized in different instruments
- Purification of metals
- Production of metals
- Batteries and cells utilized in different instruments
Quick Revision Notes
- Electrochemistry is the part of chemistry that manages the relationship between electrical energy and chemical energy and between transforming one structure into another.
- An electrochemical cell comprises two metallic electrodes dipped in electrolytic solutions. The cells are of two kinds:
(an) Electrolytic cells (b) Galvanic cells
- A galvanic cell comprises two half cells. Every half cell contains an electrolytic solution and a metallic electrode. The electrode at which-oxidation happens called an anode, and the electrode at which decrease happens is known as the cathode. The half-cells are separated from one another by methods for a permeable pot or a salt extension.
- The entry of current from one electrode to the next indicates the presence of a potential difference between them. This difference of potential, which makes the current stream from the electrode of higher negative potential, is known as the electromotive power (emf).
- Electrical energy = Quantity of electricity (coulombs) x Emf (volts)
- The potential of SHE is a self-assertive estimation of zero. E° = 0 V. It is utilized as a perspective electrode for measuring the standard electrode potentials.
- At the point when the elements are organized by their standard electrode potentials, an arrangement known as the electrochemical arrangement is gotten.
- Standard emf of a cell,
E0cell =E0cathode – E0node = E0Riglit – E0Left
- ΔG° = – nFE0cell
On the off chance that E0cell is positive, ΔG° would be negative, and the reaction would be unconstrained.The reaction would be non-unconstrained if E0 cell is Negative, ΔG° would be positive.
- An animal group with better quality decreases potential tends to acknowledge electrons to undergo a decrease or the other way around.
- The electrode’s potential in contact with its ions in solution shifts with the particle’s centralization. In this manner, for a redox reaction,
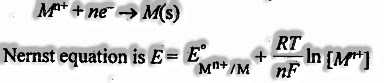
- For an electrochemical cell for which the general reaction is
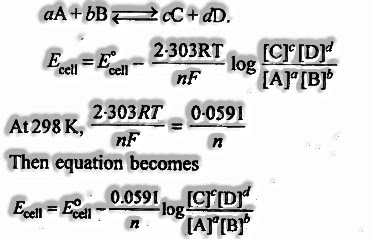
13.The balance constant, An of the cell can be identified with standard emf of a cell
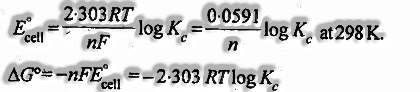
- Resistance The opposition is the proportion of obstacle to the progression of the current.
Where S = explicit opposition or resistivity. The unit of R is the ohm.
- Resistivity: Resistivity is characterized as the opposition of a conductor of 1cm length and having a territory of cross-area equivalent to 1cm2. unit of resistivity is ohm-cm
- ConductanceConductance is complementary to opposition. The unit of conductance is ohm-1 or mho or Siemen(S).
- Specific conductanceΛsp is proportional to explicit obstruction.
Specific Conductance: It is along these lines characterized as the conductance of a solution taken in a cell whose electrodes are at unit distance separated from one another and each having a region equivalent to 1 cm2. The unit of explicit conductance is ohm-1 cm-1 or S cm-1.
- Molar conductance (Λm): It is characterized as the conductance of the solution’s volume, which contains one mole of the solute and is set between two equal electrodes, which are one centimeter separated and have adequate region to hold the entire of the solution.
The unit of molar conductance is Ω-1 cm2 mol-1 or S cm2 mol-1.
Where C = grouping of the solution in moles per liter (or Molarity).
- The electrical conductance through metals diminishes with increment in temperature.
- The electrolytic conductance increments with an increment of temperature.
- Impact of Dilution on
(а) Equivalent conductance: The estimation of equivalent conductance increment with dilution and accomplishes the greatest incentive at limitless dilution.
(b) Specific conductance: The estimation of explicit conductance diminishes with dilution as the quantity of current conveying particles, i.e., ions present per cm3 of the solution, diminishes dilution.
(c) Molar conductance: The estimation of molar conductance increments with dilution lastly accomplishes the greatest incentive at unbounded dilution.
- Variety of molar conductance with focus:
(a) Strong electrolytes:

(b) Weak electrolytes:
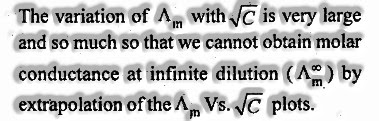
- According to Kohlrausch law According to Kohlrausch law of autonomous movement of ions, the restricting molar conductivity of an electrolyte can be communicated as the total of the contributions of the cation and the anion of the electrolyte.
Where v+, v–is the quantity of feline ions and anions per unit recipe of the electrolyte individually; λ0+, and λ0–are the restricting molar conductivities of the cation and anion separately.
- Faraday’s laws of electrolysis:
(a) First law: The measure of a substance kept or liberated at an electrode is directly corresponding to the amount of electricity that went through the electrolyte. Numerically,

where m = Mass of substance kept or liberated.
Mass of substance A kept or liberated Mass of substance B stored or liberated current in amperes t = time right away, and Z = constant called electrochemical equivalent.
(b) Second law: When a similar amount of electricity is gone through different electrolytes’ solutions, the heaviness of different substances stored or liberated at the particular electrodes is relative to their chemical equivalent weights.
26.

- The charge on one mole of electrons is around equivalent to 96500 coulombs. This amount of electricity is called Faraday constant (F).
- A battery comprises at least two galvanic cells associated with the arrangement. There are two sorts of batteries:
(a) Primary batteries: In essential batteries, when the reactants have been changed over into Products, no greater electricity is created. The cell reaction can’t be turned around, and the battery turns out to be dead.
(b) Secondary batteries: In optional batteries (or cells), the cell reaction can be turned around by going electricity through the battery (charging). It implies that the battery can be utilized over and over through an enormous number of discharging and charging cycles.
- The most widely recognized case of auxiliary batteries is the lead stockpiling battery.
- Electrical cells that are discharged to change over the energy from the burning of fills (hydrogen, carbon monoxide, methane, and so forth.) directly into the electrical energy are called power modules.
- The corrosion of metals is an electrochemical cycle. It happens in the nearness of water and oxygen.
Questions:
Ques: What is the electrode potential of the (SHE)?
Ans. The electrode potential of SHE is fixed to zero at all given temperatures.
(Electrochemistry: Class 12 )

