Chemical Bonding and Molecular Structure: Class 11 Chemistry NCERT Chapter 4
Key Features of NCERT Material for Class 11 Chemistry Chapter 4 – Chemical Bonding and Molecular Structure
In the last chapter 3, you learned about Classification of Elements and Periodicity in Properties. In this chapter, you will learn everything about Chemical Bonding and Molecular Structure.
Things being the way they are, the reason do the reactions happen as they happen? Would you be able to blend just hydrogen and oxygen in a gas chamber and get the water that you drink? No! This is the reason as to why we are going to take a gander at the basics of compound bonding in this part. We will initially comprehend what substance bonding is. At that point, we will see its sorts, and the sky’s the limit from there.
What is Chemical Bonding?
For what reason do different elements or substances experience a transformation under a lot of conditions? As understudies of chemistry, we should realize that. The response to this fascinating question lies in the section of the basics of chemical bonding.
Even a small entity in this universe tries to get steady. This happens just by losing energy. In an interaction between two sorts of issues, one applies a force on another. The energies between the two kinds of issues rely upon the idea of the force between them. In the event that the force is alluring, the energy diminishes. Then again, on the off chance that it’s horrible, the energy increments at that point.
In situations where the force is alluring, the two atoms get bound together. Such a force is the thing that we call a concoction bond. Accordingly, we can characterize substance bonding as follows: “The alluring force which holds different constituents (particle, ions, and so forth.) together and settles them by the general loss of energy is concoction bonding.”
Quick revision notes
-
Chemical Bond
The force that holds different atoms in a molecule is called a compound bond.
-
Octet Rule
Atoms of different elements participate in concoction combinations so as to finish their octet or to accomplish the respectable gas configuration.
-
Valence Electrons
It is the furthest shell electron which participates in compound combination.
- Facts Stated by Kossel in Relation to Chemical Bonding
— In the periodic table, the profoundly electronegative halogens and the exceptionally electro-positive alkali metals are isolated by noble gases.
— The formation of an anion and cation by the halogens and alkali metals are shaped by the increase of electrons and the loss of electrons separately.
— Both the negative and positive ions obtain the honorable gas configuration.
— The negative and positive ions are settled by electrostatic attraction Example,
-
Modes of Chemical Combination
The transfer of electrons: The concoction bond shaped by the total transfer of at least one electron, starting with one particle, then onto the next, is named an electrovalent bond or ionic bond.
— The bond which is framed by the equivalent sharing of electrons between a couple of atoms is called a covalent bond. In these bonds, electrons are contributed by both.
— Co-ordinate bond: When the electrons are contributed by one molecule and shared by both, the bond is framed, and it is known as a dative bond or co-ordinates bond.
-
Ionic or Electrovalent Bond
Ionic or Electrovalent bond is shaped by the total transfer of electrons starting with one iota then onto the next. By and large, it is framed among metals and non-metals. We can say that it is the electrostatic force of attraction that holds the oppositely charged ions together.
The compounds which are framed by the ionic or electrovalent bond are known as electrovalent compounds. For Example,
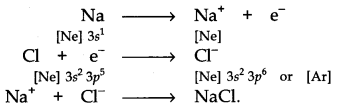
(I) NaCl is an electrovalent compound. Formation of NaCl is given beneath:
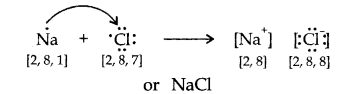
Na+ ion has the configuration of Ne while Cl–ion speaks to the configuration of Ar.
(ii) Magnesium and oxygen were yielding magnesium oxide.
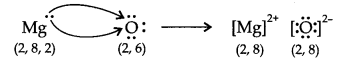
Electrovalency: Electrovalency is the quantity of electrons lost or picked up during the formation of an ionic bond or electrovalent bond.
-
Factors Affecting the Formation of Ionic Bond
(I) Ionization enthalpy: As we realize that ionization enthalpy of any element is the measure of the energy required to expel an electron from the peripheral shell of an isolated vaporous molecule to change over it into cation.
Henceforth, the less the ionization enthalpy, the simpler the formation of a cation will be and a more prominent opportunity to frame an ionic bond. Because of this explanation, alkali metals tend to shape an ionic bond.
For instance, in formation of Na+ ion I.E = 496 kJ/mol
If there should arise an occurrence of magnesium, it is 743 kJ/mole. That is the reason the formation of positive ions for sodium is more straightforward than that of magnesium.
In this manner, we can reason that bringing down the ionization enthalpy, more prominent the odds of ionic bond formation.
(ii) Electron gain enthalpy (Electron affinities): It is characterized as the energy delivered when an isolated vaporous particle takes up an electron to frame anion. More prominent the negative electron gain enthalpy, simpler will be the formation of the anion. Thus, the likelihood of the formation of ionic bond increments.
For instance. Halogens have a high electron affinity. Thus, the formation of the anion is basic in halogens.
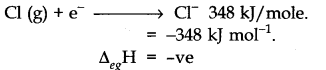
(iii) Lattice energy or enthalpy: It is characterized as the measure of the energy required to isolate 1 mole of an ionic compound into discrete oppositely charged ions.
The lattice energy of an ionic compound relies on the following variables:
(I) Size of the ions: Smaller the size, more prominent will be the lattice energy.
(ii) Charge on the ions: Greater the extent of charge, more prominent the interionic attraction, and consequently higher the lattice energy.
-
General Characteristics of ionic Compounds
(I) Physical State: They, for the most part, exist as glasslike solids, known as a precious stone lattice. Ionic compounds don’t exist as single molecules like different vaporous molecules, e.g., H2, N2, O2, Cl2, etc.
(ii) Melting and boiling focus: Since ionic compounds contain high interionic force between them, they, by and large, have high melting and boiling focuses.
(iii) Solubility: They are solvent in polar solvents, for example, water yet don’t dissolve in organic solvents like benzene, CCl4etc.
(iv) Electrical conductivity: In the strong state, they are helpless conveyors of electricity yet in the liquid state, or when dissolved in water, they lead electricity.
(v) Ionic reactions: Ionic compounds produce ions in the solution, which gives exceptionally quick reaction with oppositely charged ions.
For instance,
![]()
-
Covalent Bond—Lewis-Langmuir Concept
At the point when the bond is framed between at least two atoms by common contribution and sharing of electrons, it is known as a covalent bond. On the off chance that the consolidating atoms are the same, the covalent molecule is known as homoatomic. In the event that they are different, they are known as a heteroatomic molecule.
For Example,
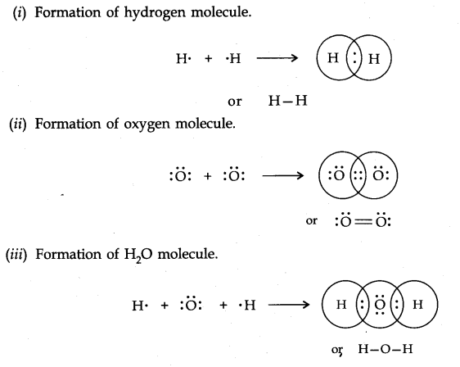
iM.
-
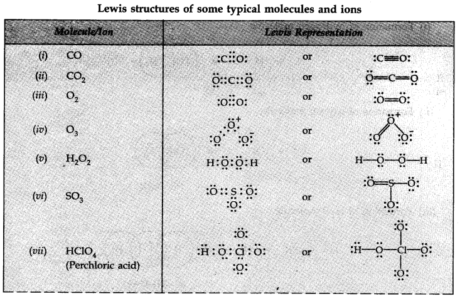
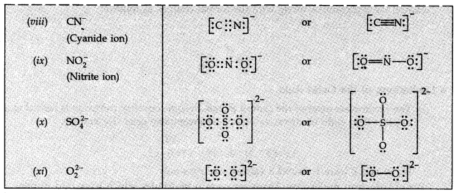
Lewis Representation of Simple Molecules (the Lewis Structures)
The Lewis speck Structure can be composed through the accompanying advances:
(I) Calculate the all outer number of valence electrons of the joining atoms.
(ii) Each anion implies the addition of one electron, and every cation implies evacuation of one electron. This gives the complete number of electrons to be distributed.
(iii) By knowing the concoction images of the consolidating atoms.
(iv) After setting shared pairs of electrons for a single bond, the rest of the electrons may represent either different bonds or as lone pairs. It is to be noticed that the octet of every particle ought to be finished.
-
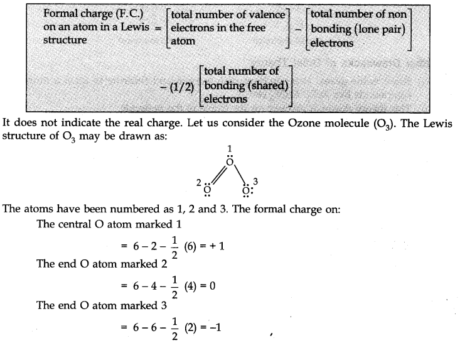
Formal Charge
In polyatomic ions, the net charge is the charge on the ion all in all and not by a specific iota. In any case, charges can be appointed to individual atoms or ions. These are called formal charges.
It very well may be communicated as
- Limitations of the Octet Rule
(I) The inadequate octet of the focal atoms: In some covalent compounds the focal molecule has under eight electrons, i.e., it has a fragmented octet. For instance,
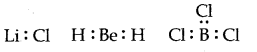
Li, Be, and B has 1, 2, and 3 valence electrons in particular.
(ii) Odd-electron molecules: There are sure molecules that have an odd number of electrons. The octet rule isn’t applied for all the atoms.
![]()
(iii) The extended Octet: In numerous compounds, there are in excess of eight valence electrons around the focal particle. It is named as an extended octet. For Example,

- Other Drawbacks of Octet Theory
(I) Likewise, some respectable gases consolidate with oxygen and fluorine to frame various compounds like XeF2, XeOF2, and so on.
(ii) This hypothesis doesn’t represent the state of the molecule.
(iii) It doesn’t give any thought regarding the energy of The molecule and relative security.
-
Bond Length
It is characterized as the balance distance between the focuses of the nuclei of the two bonded atoms. It is communicated as far as A. Tentatively, it very well may be characterized by X-beam diffraction or electron diffraction technique.
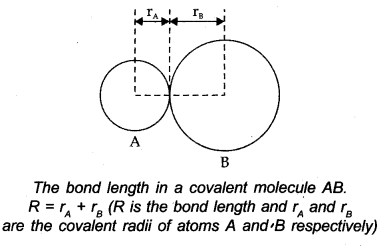
-
Bond Angle
It is characterized as – the angle between the lines speaking to the orbitals containing the bonding – electrons.
It encourages us to fit as a fiddle. It tends to be communicated in degrees. Spectroscopic strategies can tentatively dictate the bond angle.
-
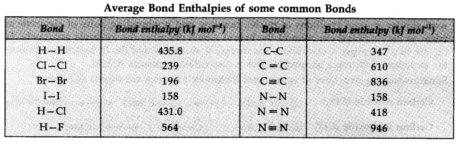
Bond Enthalpy
It is characterized as the measure of the energy required to break one mole of bonds of a specific kind to isolate them into vaporous atoms.
Bond Enthalpy is otherwise called bond dissociation enthalpy or basic bond enthalpy. Unit of bond enthalpy = kJ mol-1
The size of the bond enthalpy is also identified with bond multiplicity. More prominent the bond multiplicity more will be the bond enthalpy. E.g., bond enthalpy of the C — C bond is 347 kJ mol-1 while that of C = C bond is 610 kJ mol-1.
In polyatomic molecules, the term mean or normal bond enthalpy is used.
- Bond Order
According to Lewis, in a covalent bond, the bond request is given by the quantity of bonds between two atoms in a molecule. For example,
A bond request of H2 (H — H) =1
Bond request of 02 (O = O) =2
Bond request of N2 (N = N) =3
Isoelectronic molecules and ions have indistinguishable bond orders. For example, F2 and O22-have bond request = 1. N2, CO, and NO+ have bond request = 3. With the increase in a bond request, bond enthalpy increases, and bond length decreases. For example,
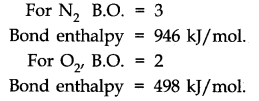
-
Resonance Structures
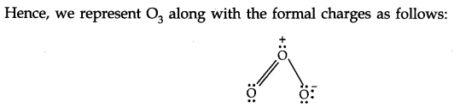
There are numerous molecules whose conduct can’t be explained by a single-Lewis structure, Tor example, Lewis structure of Ozone represented as follows:
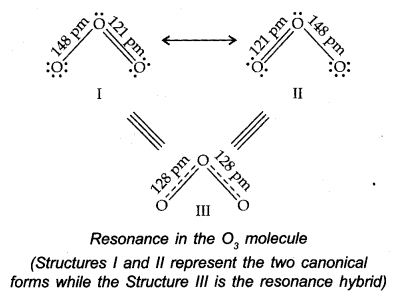
Thus, according to the concept of resonance, at whatever point a single Lewis structure can’t explain all the molecule’s properties, the molecule is supposed to have numerous structures with similar energy. The positions of nuclei, bonding, and nonbonding pairs of electrons are taken as the standard structure of the hybrid, which precisely describes the molecule. For 03, the two structures shown above are sanctioned structures, and the III structure represents the structure of 03 all the more precisely. This is also called a resonance hybrid.
Some resonating structures of some more molecules and ions are shown as follows:
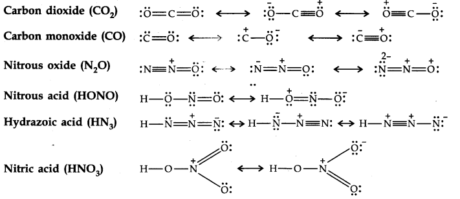
-
Polarity of Bonds
Two atoms similarly pull in Nonpolar Covalent bonds: When the atoms joined by the covalent bond are the same as; H2, 02, Cl2, the shared pair of electrons, and thus the shared electron pair is equidistant to the two.
Then again, we can say that it lies precisely in the focal point of the bonding atoms. As a result, no poles are developed, and the bond is called a nonpolar covalent bond. The corresponding molecules are known as nonpolar molecules.
For Example,
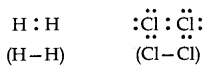
When covalent bonds are framed between different atoms of different electronegativity, shared electron pairs between two atoms get displaced towards exceptionally electronegative atoms.
For Example, in HCl molecule, since electronegativity of chlorine is high as compared to hydrogen thus, electron pair is displaced more towards chlorine particle; thus chlorine will obtain a partial negative charge (δ–), and hydrogen iota have a partial positive charge (δ+) with the size of charge same as on chlorination. Such a covalent bond is called a polar covalent bond.
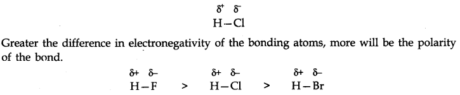
-
Dipole Moment
Because of polarity, polar molecules are also known as dipole molecules, and they possess dipole second. Dipole second is characterized as the product of the size of the positive or negative charge and the distance between the charges.

-
Applications of Dipole Moment
(I) For deciding the polarity of the molecules.
(ii) In finding the shapes of the molecules.
For example, the molecules with zero dipoles second will be linear or symmetrical. Those molecules which have asymmetrical shapes will be either twisted or precise.
(e.g., NH3 with μ = 1.47 D).
(iii) In ascertaining the percentage ionic character of polar bonds.
- The Valence Shell Electron Pair Repulsion (VSEPR) Theory
Sidgwick and Powell in 1940 proposed a simple hypothesis based on the repulsive character of electron pairs in the atoms’ valence shell. It was additionally developed by Nyholm and Gillespie (1957).
Fundamental Postulates are the accompanying:
(I) The specific shape of the molecule depends upon the quantity of electron pairs (bonded or non bonded) around the focal atoms.
(ii) The electron pairs keep to repel each other when they exist around the focal particle, and the electron clouds are contrarily charged.
(iii) Electron pairs attempt to take such a position, which can limit the repulsion between the electrons.
(iv) The shape of the valence shell is considered as a sphere having electron pairs placed at the most extreme distance.
(v) Multiple bonds are treated as the off chance that it is a single electron pair and the electron pairs that constitute the bond as single pairs.
-
Valence Bond Theory
Valence bond hypothesis was presented by Heitler and London (1927) and developed by Pauling and others. It is based on the concept of atomic orbitals and the electronic configuration of the atoms.
Let us consider the formation of hydrogen molecules based on the valence-bond hypothesis.
Let two hydrogen atoms A and B have their nuclei NA and NB, and electrons present in them are eA and eB.
As these two atoms come closer new alluring and repulsive forces start to operate.
(I) The nucleus of one particle is pulled in towards its electron and the electron of the other and the other way around.
(ii) Repulsive forces arise between the electrons of two atoms and nuclei of two atoms. In general, appealing forces will bring the two atoms closer, whereas repulsive forces will, in general, push them apart.
-
Orbital Overlap Concept
According to the orbital overlap concept, covalent bond shared between atoms results in the overlap of orbitals having a place with the atoms having opposite spins of electrons. Formation of the hydrogen molecule as a result of the overlap of the two atomic orbitals of hydrogen atoms is shown in the following figures:
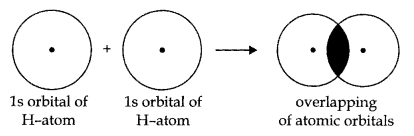
The stability of a Molecular orbital depends upon the degree of the overlap of the atomic orbitals.
-
Types of Orbital Overlap
Depending upon the type of overlapping, the covalent bonds are of two types, known as sigma (σ ) and pi (π) bonds.
(I) Sigma (σ bond): Sigma bond is shaped by the start to finish (head-on) overlap of bonding orbitals along the internuclear axis.
The pivotal overlap, including these orbitals, is of three types:
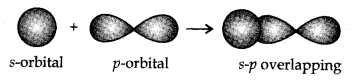
- s-s overlapping: In this case, there exist an overlapping of two half-filled s-orbitals along the internuclear axis, as shown beneath:
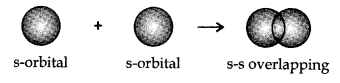
- s-p overlapping:This type of overlapping occurs between half-filled s-orbitals of one particle and half-filled p-orbitals of another atom.

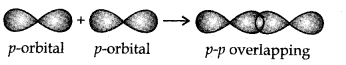
- p-p overlapping:This type of overlapping takes place between half-filled p-orbitals of the two approaching atoms.
.
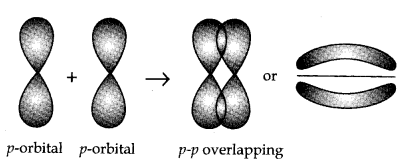
(ii) pi (π bond): π bond is framed by the atomic orbitals when they overlap in such a manner that their axes stay parallel to one another and perpendicular to the internuclear axis. The orbital shape is because of horizontal overlapping or sidewise overlapping.
- Strength of Sigma and pi Bonds
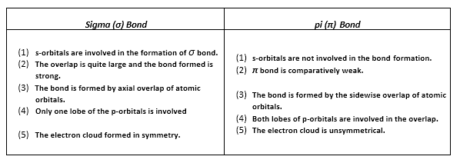
The Sigma bond (σ bond) is framed by the hub overlapping of the atomic orbitals while the π-bond is shaped by the sidewise overlapping. Since pivotal overlapping is more prominent as compared to sidewise. Thus, the sigma bond is said to be a stronger bond in comparison to a π-bond.
The distinction between sigma and pi bonds
-
Hybridisation
Hybridization is the intermixing of the orbitals of slightly different energies to redistribute their energies resulting in the formation of a new set of orbitals of identical energies and shape.
Salient Features of Hybridisation:
(I) Orbitals with almost equivalent energy participate in hybridization.
(ii) Number of hybrid orbitals produced is equivalent to the number of atomic orbitals blended,
(iii) The type of hybridization can indicate the geometry of a covalent molecule.
(iv) The hybrid orbitals are more successful in shaping stable bonds than the pure atomic orbitals.
Conditions necessary for hybridization:
(I) Orbitals of valence shells participate in the hybridization.
(ii) Orbitals engaged with hybridization should have almost equivalent energy.
(iii) The promotion of electrons is not a necessary condition prior to hybridization.
(iv) In some cases, filled orbitals of valence shells also participate in hybridization.
Types of Hybridisation:
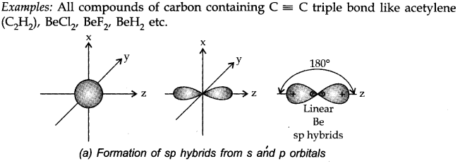
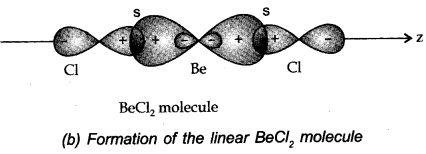
(I) sp hybridization: When one s and one p-orbital hybridize to frame two equal orbitals, the orbital is known as sp hybrid orbital. The type of hybridization is called sp hybridization.
Every one of the hybrid orbitals shaped has half s-character and half, p-character. This is also known as diagonal hybridization.
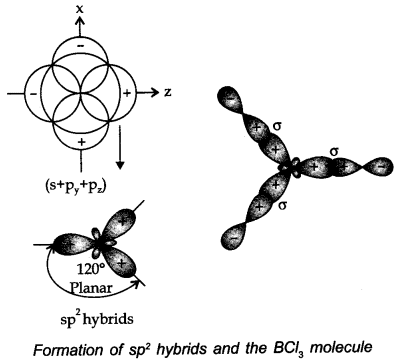
(ii) sp2 hybridisation: In this type, one s and two p-orbitals hybridize to frame three proportionate sp2 hybridized orbitals.
All three hybrid orbitals stay in the same plane, creating an angle of 120°. Example. A couple of compounds wherein sp2 hybridization occurs are BF3, BH3, BCl3 carbon compounds containing a double bond, and so forth.
(iii) sp3 hybridisation: In this type, one s and three p-orbitals in the valence shell of a particle get hybridized to shape four proportional hybrid orbitals. There is 25% s-character and 75% p-character in each sp3 hybrid orbital. The four sp3 orbitals are directed towards four corners of the tetrahedron.
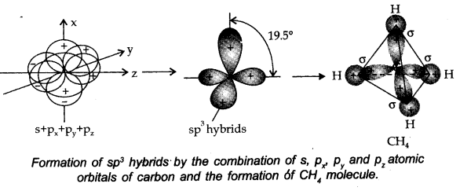
The angle between the sp3 hybrid orbitals is 109.5°.
A compound where sp3 hybridization occurs is, (CH4). The structures of NH2 and H20 molecules can also be explained with the help of sp3 hybridization.
-
Formation of Molecular Orbitals: Linear Combination of Atomic Orbitals (LCAO)
The linear combination of atomic orbitals can explain the formation of molecular orbitals. A combination takes place either by addition or by subtraction of wave function, as shown underneath.
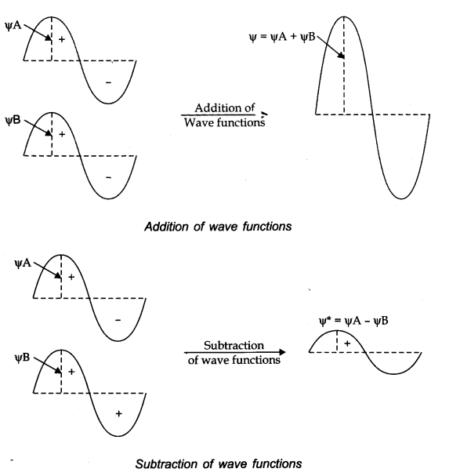
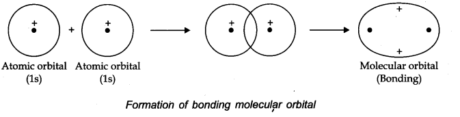
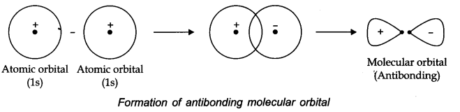
The molecular orbital framed by the addition of atomic orbitals is called bonding molecular orbital, while molecular orbital shaped by subtraction of atomic orbitals is called an antibonding molecular orbital.
Conditions for the combination of atomic orbitals:
(1) The consolidating atomic orbitals must have almost equivalent energy.
(2) The consolidating atomic orbitals must have the same symmetry about the molecular axis.
(3) The consolidating atomic orbitals must overlap to the most extreme degree.
-
Types of Molecular Orbitals
Sigma (σ) Molecular Orbitals: Around the bond-axis, They happen to be symmetrical.
Pi (π) Molecular Orbitals: Because of the presence of positive lobes above and negative lobes underneath the molecular plane, they are not symmetrical underneath the molecular plane.
- Electronic configuration and Molecular Behavior
The distribution of electrons amongst various molecular orbitals is called the electronic configuration of the molecule.
- Stability of Molecules

- Bond Order
The bond request is characterized as half of the difference between the quantity of electrons present in bonding and antibonding molecular orbitals.
Bond request (B.O.) = 1/2 [Nb-Na]
The bond request might be an entire number, a fraction, or even zero.
It might also be positive or negative.
Nature of the bond: Integral bonds request an incentive for the single twofold, and triple bonds will be 1, 2, and 3, respectively.
Bond-Length: Bond request is inversely proportional to bond-length. Thus, more prominent the bond request, smaller will be the bond-length.
Attractive Nature: If all the molecular orbitals have paired electrons, the substance is diamagnetic. In the event that at least one molecular orbitals have unpaired electrons, it is paramagnetic, e.g., 02 molecule.
-
Bonding in Some Homonuclear (Diatomic) Molecules
(1) Hydrogen molecule (H2): The combination of two hydrogen atoms frames it. Every hydrogen iota has one electron in Is orbital, so, the electronic configuration of the hydrogen molecule is
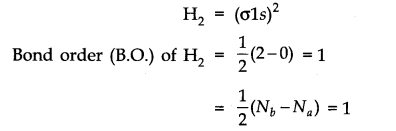
This indicates single covalent bond bonds two hydrogen atoms. The bond dissociation energy of hydrogen has been found = 438 kJ/mole. Bond-Length = 74 pm
No unpaired electron is present in this manner, and it is diamagnetic.
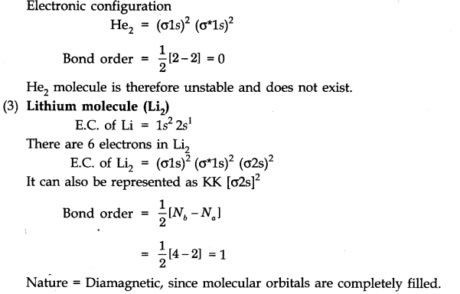
(2) Helium molecule (He2): Each helium particle contains 2 electrons, thus in the He2 molecule, there would be 4 electrons.
The electrons will be obliged in σ1s and σ*1s molecular orbitals:

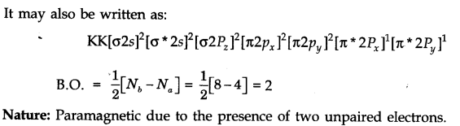
-
Hydrogen Bonding
When exceptionally electronegative elements like nitrogen, oxygen, fluorine are appended to hydrogen to shape covalent bonds, the electrons of the covalent bond are shifted towards the more electronegative particle. Thus, a partial positive charge develops on a hydrogen particle, forming a bond with the other electronegative molecule. This bond is known as hydrogen bond, and it is more vulnerable than the covalent bond. For example, in HF molecules, hydrogen bonds exist between hydrogen particles of one molecule and fluorine iota of another molecule.
It tends to be depicted as
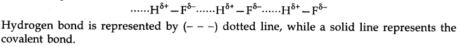
- Types of H-Bonds
(I) Intermolecular hydrogen bond (ii) Intramolecular hydrogen bond.
(I) Intermolecular hydrogen bond: It is framed between two different molecules of the same or different compounds, for Example, in HF molecules, water molecules, etc.
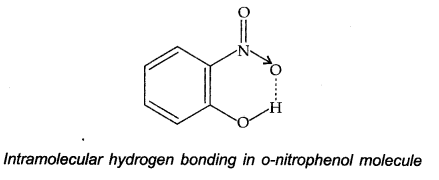
(ii) Intramolecular hydrogen bond: In this type, a hydrogen particle is in the middle of the two profoundly electronegative F, N, O atoms present inside the same molecule. For example, in o-nitrophenol, the hydrogen is in the middle of the two oxygen atoms.
Questions
Q: What are London dispersion forces?
Ans: London dispersion forces are those that happen because of the temporary imbalances of charge occurring inside a particle. The concentration of charge of the atoms undergoes constant changes as these electrons are always in motion. This creates a temporary shift in the general charge distribution of the particle.
At the point when this particle happens to interact with another, the temporary awkwardness of charge will result in an attraction of positive and negative charges. These are the London dispersion forces.