Aldehydes, Ketones And Carboxylic Acids: Class 12 Chemistry Chapter 12

Key Features of NCERT Material for Class 12 Chemistry Chapter 12 – Aldehydes, Ketones And Carboxylic Acids
In the previous chapter: Alcohol, Phenols and Ethers, we studied chemical and physical properties of alcohol, phenol and ethers. We realize that organic compounds (Ketones and aldehydes) production is conceivable in mechanical scale and research centre scale. Nonetheless, do you realize that ketone, just as aldehyde production, happens normally in many living beings? Ketone generation as ribulose-1,5-bisphosphate is one of the means of photosynthesis and help in the formation of the fundamental organic compounds during photosynthesis.
Ketones are available as sugars and are called ketoses. It is additionally present in many vertebrates including people as ketone bodies. Presently, that you know how living beings produce ketone, take a stab at discovering how living beings create aldehydes. In this subject, we will figure out how the preparation of Aldehydes and Ketones is conceivable by different chemical reactions.
Aldehydes and Ketones
Aldehydes and Ketones are basic organic compounds containing a carbonyl gathering. Carbonyl gathering contains carbon-oxygen twofold bond. These organic compounds are straightforward on the grounds that the carbon atom presents in the carbonyl gathering need responsive gatherings, for example, OH or Cl.
Quick revision notes
Aldehydes:
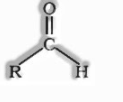
They are the organic compounds where carbonyl gathering is appended to one hydrogen atom and one alkyl or aryl gathering.
Where R can be an alkyl or aryl gathering
Preparation of aldehydes:
a) By oxidation of alcohols: Oxidation of primary alcohols in the nearness of oxidizing agent like K2Cr2O7/H2SO4, KMnO4, CrO3 gives aldehydes.
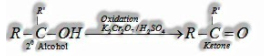
b) By dehydrogenation of alcohols: When the fumes of primary alcohol went through warmed copper at 573 K, it structures aldehyde.
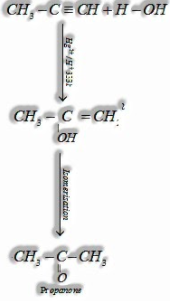
c) By hydration of alkynes: Ethyne on hydration with at 333 K structures acetaldehyde.
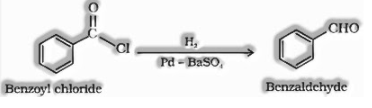
d) By Rosenmund reduction: Hydrogenation of acyl chloride over palladium on barium sulfate gives aldehyde.

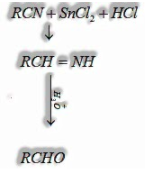
e) By reduction of nitriles:
I) Stephen Reaction: Reduction of nitriles in the nearness of stannous chloride in the nearness of HCl gives imine which on hydrolysis gives matching aldehyde.

ii) Nitriles are specifically decreased by DIBAL-H (Diisobutylaluminium hydride) to aldehydes.
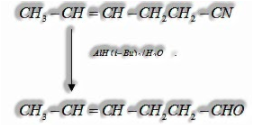
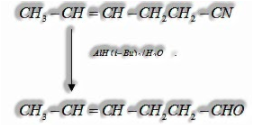
f) By reduction of ester: Esters are decreased to aldehydes in the nearness of DIBAL-H (Diisobutylaluminium hydride)
g) From Hydrocarbons:
(I) By oxidation of methylbenzene:
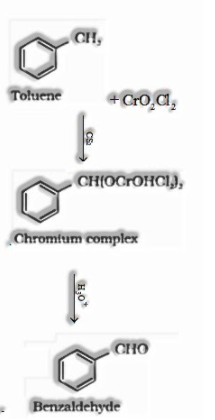

Etard Reaction: Chromyl chloride oxidizes methyl gathering to a chromium complex, which on hydrolysis gives comparing benzaldehyde.
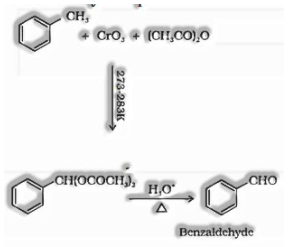
Utilizing chromium oxide: Toluene or subbed toluene is changed over to benzaldehyde in the nearness of chromic oxide in acidic anhydride.

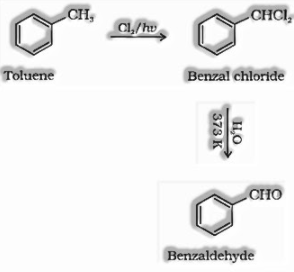
(ii) Through side-chain chlorination succeeded by hydrolysis: Halogenation of toluene: Sidechain halogenation of toluene gives benzal chloride which on hydrolysis gives Benzaldehyde.

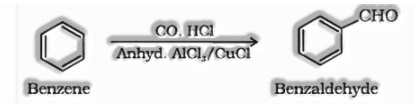
(iii) Gatterman – Koch reaction: Benzene or its subsidiaries after treatment by carbon monoxide and HCl in the nearness of anhydrous aluminium chloride or cuprous chloride (CuCl) produces benzaldehyde or substituted benzaldehydes.

Ketones:
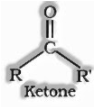
Ketones are the organic compounds wherein carbonyl gathering is appended to two alkyl gathering or aryl gathering or both alkyl and aryl gathering.
Where R, R’ might be alkyl or aryl.
Preparation of ketones:
a) By oxidation of alcohols: Oxidation of secondary alcohols in the nearness of oxidizing agent, gives ketones.
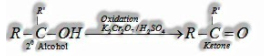
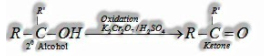
b) By dehydrogenation of alcohols: When the fumes of secondary alcohol are ignored warmed copper at 573 K, dehydrogenation happens and a ketone is framed.
c) By hydration of alkynes: Alkynes on hydration with at 333 K structure ketones.



d) From acyl chloride: Acyl chloride on treatment with dialkyl cadmium (arranged by the reaction of cadmium chloride with Grignard reagent) gives ketone.
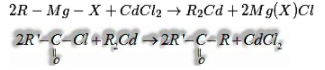
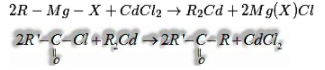
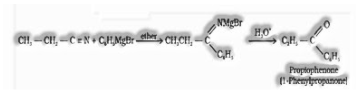
e) From nitriles: On treatment with Grignard reagent succeeded by hydrolysis give ketones.

f) By Friedel Crafts acylation reaction: Benzene or subbed benzene on treatment with an acid chloride in the nearness of anhydrous aluminium chloride structures ketone.


g) Preparation of aldehydes and ketones through ozonolysis of alkenes:
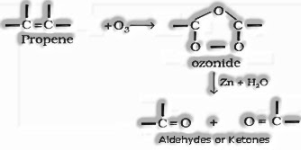

Reactions of aldehydes and ketones:
Aldehydes are far more reactive in contrast to ketones in nucleophilic addition reactions due to steric and electronic reasons (or inductive impact).
Electronic Effect:
Relative reactivities of aldehydes and ketones in nucleophilic addition reactions is because of the positive charge on carbonyl carbon. More positive charge implies more reactivity. Electron delivering intensity of two alkyl bunches in ketones is more than one in the aldehyde. Along these lines, a positive charge is decreased in ketones when contrasted with aldehydes. Along these lines, ketones are less reactive than aldehydes.
Stearic Effect:
As the number and size of alkyl bunch increments, the block to the assault of nucleophile likewise increments and reactivity diminish. In aldehydes, there is one alkyl gathering and one hydrogen atom, though in ketones there are two alkyl gatherings (same or extraordinary).
Nucleophilic addition reactions of aldehydes and ketones:
(a) Addition of hydrogen cyanide (HCN) to shape cyanohydrins
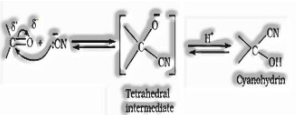

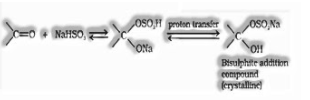
(b) Addition of sodium hydrogen sulphite to structure bisulphate addition compound

(c) Addition of Grignard reagent (RMgX) to shape alcohol
![]()
![]()
Addition of alcohol:
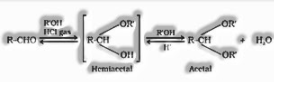

(I) Aldehydes on the addition of monohydric alcohol in the nearness of dry HCl structures hemiacetal and acetal.
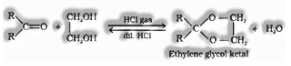
(ii) Ketones don’t respond with monohydric alcohols. Ketones respond with ethylene glycol under comparable conditions to frame cyclic items known as ethylene glycol ketals.

Addition of smelling salts and its subordinates:
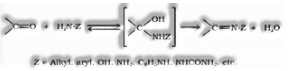
Reduction of aldehydes and ketones:
(a) Reduction to alcohols:
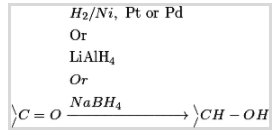

Aldehydes and ketones on synergist hydrogenation in the nearness of Ni, Pt or Pd by utilizing lithium aluminium hydride or sodium borohydride structures primary and secondary alcohols individually.
(b) Reduction to hydrocarbons:
(I) Clemmensen reduction:
Carbonyl gathering of aldehydes and ketones is diminished to aggregate on treatment with zinc amalgam and concentrated hydrochloric acid.
(ii) Wolff-Kishner reduction:
Carbonyl gathering of aldehydes and ketones is decreased to amass on treatment with hydrazine followed by warming with sodium or potassium hydroxide in high boiling dissolvable, for example, ethylene glycol.
(iii) 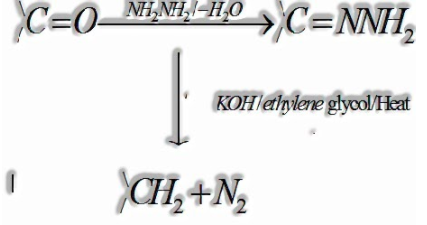

Oxidation of aldehydes and ketones:
(I) Aldehydes are oxidized to acids in the nearness of gentle oxidizing specialists HNO3, K2Cr2O7, KMnO4.
(ii) Ketones are oxidized under extreme conditions for example with ground-breaking oxidizing operators like ![]()
![]()
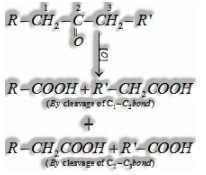
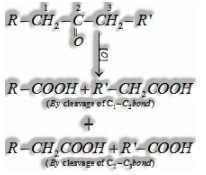
In the event of unsymmetrical ketones cleavage happens so that keto group remains with littler alkyl gathering. This is known as Popoff’s rule.
(iii)Haloform reaction: Aldehydes and ketones having, in any event, one methyl group connected to the carbonyl carbon atom for example methyl ketones are oxidized by sodium hypohalite to sodium salts of matching carboxylic acids having one carbon atom less of the carbonyl compound. The methyl bunch is changed over to haloform.
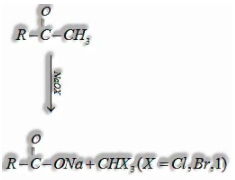
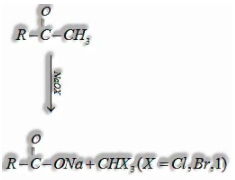
Reactions of aldehydes and ketones because of – hydrogen:
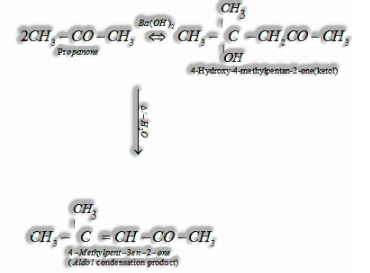
(I) Aldol condensation: Aldehydes and ketones having, in any event, one – hydrogen experience a self-condensation within the sight of dilute alkaline as a catalyst to frame – hydroxy aldehydes (aldol) or – hydroxy ketones (ketol), individually.
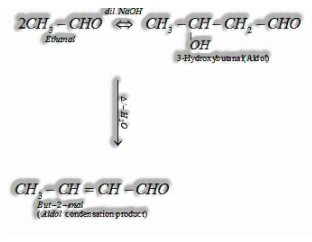


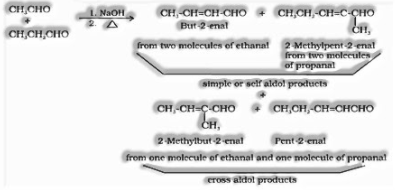
(ii) Cross aldol condensation: Aldol condensation between two distinct aldehydes and ketones is called aldol condensation. On the off chance that they two contain – hydrogen atoms, it gives a blend of four items.

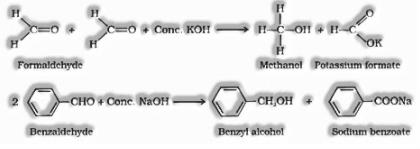
Cannizzaro reaction: Aldehydes which don’t have a – hydrogen atom goes through self-oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali to shape alcohol and salt of the acid.

Test to identify aldehydes and ketones:
Tollen’s test:
When an aldehyde is warmed with Tollen’s reagent it structures a silver mirror. Tollen’s reagent is the ammoniacal solution of
Ketones don’t frame silver mirror and thus don’t give this test.
Fehling’s test:
When an aldehyde is warmed with Fehling’s reagent it forms reddish brown coloured precipitates of cuprous oxide.Fehling’s reagent: Fehling solution A (watery solution of ) + Fehling solution B (soluble solution of sodium-potassium tartarate)
Ketones don’t give this test.
Carboxylic Acids:
Carboxylic acids are the compounds containing the carboxyl functional group (- COOH).
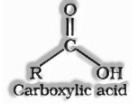
Preparation of carboxylic acid:
(I) From alcohols:
Primary alcohols are promptly oxidized to carboxylic acids with normal oxidizing agents, for example, potassium permanganate in neutral, acidic or alkaline media or by potassium dichromate (K2Cr2O7) and chromium trioxide in acidic media.
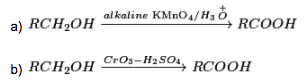

(ii) From aldehydes:
The Oxidation of aldehydes in the vicinity of mild oxidizing agents like Tollen’s reagent (ammoniacal solution of ) or Fehling reagent (Fehling solution A (fluid solution of ) + Fehling solution B (watery solution of sodium-potassium tartarate)) structures carboxylic acids.
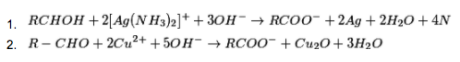
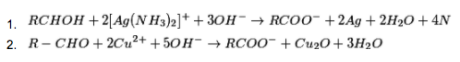
(iii) From alkylbenzenes:
Aromatic carboxylic acids can be set up by incredible oxidation of alkylbenzenes with chromic acid or acidic or basic potassium permanganate.
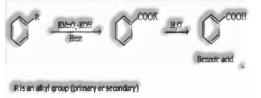

(iv) From alkenes:
Suitably subbed alkenes are oxidized to carboxylic acids on oxidation with acidic potassium permanganate or acidic potassium dichromate.
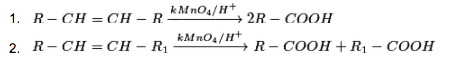
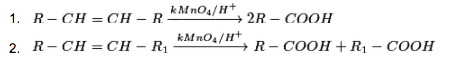
(v) From Nitriles:
Nitriles on hydrolysis in the nearness of weakening acids or bases structures amide which on further hydrolysis gives carboxylic acid.
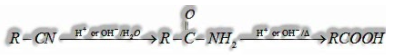
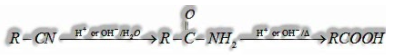
(vi) From Grignard reagent:
Grignard reagents respond with carbon dioxide (dry ice) to frame salts of carboxylic acids which on hydrolysis structures carboxylic acids.
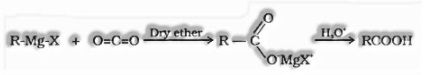
(vii) From acyl halides and anhydrides:
Acid chlorides, when hydrolysed with water, produce carboxylic acids. On primary hydrolysis, carboxylate ions are framed which on further acidification structures relating carboxylic acids. Anhydrides on hydrolysis structures relating acid(s)
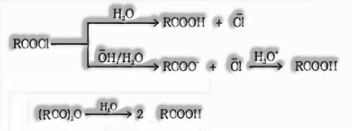

(viii) From esters:
Acidic hydrolysis of esters gives legitimately carboxylic acids while essential hydrolysis gives carboxylates, which on acidification give comparing carboxylic acids.
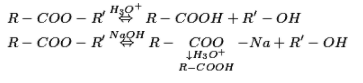
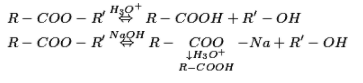
Physical properties of carboxylic acids:
(I) Solubility: As the size of alky bunch builds solubility of carboxylic acid declines in light of the fact that non-polar part of the acid increments
(ii) Boiling points: Carboxylic acids are greater boiling fluids than aldehydes, ketones and even alcohols of identical molecular masses. This is because of the broad association of carboxylic acid molecules through intermolecular hydrogen bonding.
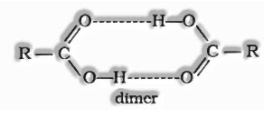

The acidity of carboxylic acids:
Carboxylic acids are more acidic than phenols. The quality of acid relies upon the degree of ionization which thusly relies upon the strength of anion framed.
(I) Effect of electron giving substituents on the acidity of carboxylic acids: Electron giving substituent diminishes steadiness of carboxylate ion by heightening the negative charge and henceforth diminishes acidity of carboxylic acids.
(ii) Effect of electron pulling back substituent on the acidity of carboxylic acids: Electron pulling back gathering builds the strength of carboxylate ion by delocalizing negative charge and henceforth, expands acidity of carboxylic acid. The impact of the accompanying gatherings in expanding acidity request is Ph< I < Br <cl< f=””>2 < CF3</cl<>
(an) Effect of number of electron pulling back gatherings: As the quantity of electron pulling back gatherings increments – I impact increments, expanding the acid quality
(b) Effect of position of electron pulling back gathering: As the separation between electron pulling back gathering and carboxylic gathering expands, electron pulling back impact diminishes.
The reaction of carboxylic acids:
Reactions including cleavage of C-OH bond:
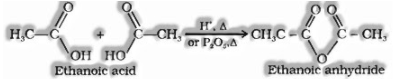
Carboxylic acids on warming with mineral acids, for example, or with giving comparing anhydride.
(I) Anhydride formation:

(ii) Esterification: Carboxylic acids are esterified with alcohols within the sight of a mineral acid, for example, concentrated or HCl gas as an impetus.
(iii) Carboxylic acids respond with PCl5, PCl3 and SOCl2 to frame acyl chlorides.
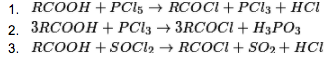
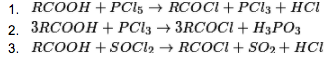
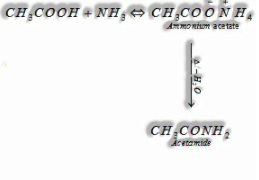
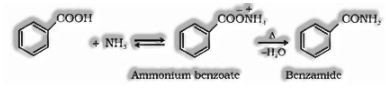
(iv) Reaction with alkali (NH3): Carboxylic acids respond with smelling salts to give ammonium salt which on further warming at high temperature gives amides.
Reactions including COOH gathering:
(I) Reduction: Carboxylic acids are decreased to alcohols in the nearness of LiAlH4 or B2H6.
![]()
![]()
(ii) Decarboxylation: Sodium or potassium salts of carboxylic acids on warming with soft drink lime (NaOH + CaO in the proportion of 3:1) gives hydrocarbons which contain one carbon not exactly the parent acid.
Reactions including substitution reaction in hydrocarbon part:
(I) Hell-Volhard-Zelinsky reaction: Carboxylic acids having a – hydrogen are halogenated at the – position on treatment with chlorine or bromine within the sight of a modest quantity of red phosphorus to give – halocarboxylic acids)
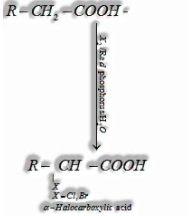

(ii) Ring substitution in aromatic acids: Aromatic carboxylic acids experience electrophilic substitution reactions. Carboxyl gathering in benzoic acid is electron pulling back gathering and is meta coordinating.
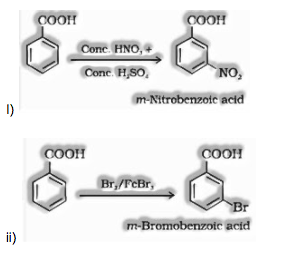
Questions
Recognize and name the reagents that help in doing the accompanying reactions
Cyclohexanol to cyclohexanone-
Hexan-1-ol to hexanal
Be that as it may, 2-ene to ethanol
Allyl alcohol to propenal
Solution:
Anhydrous CrO3
C5H5NH+CrO3Cl (PCC)
O3/H2O-Zn dust
PCC