Alcohols, Phenols And Ethers : Class 12 Chemistry NCERT Chapter 11

Key Features of NCERT Material for Class 12 Chemistry Chapter 11 – Alcohols, Phenols And Ethers
In the previous chapter, Haloalkenes and Haloarenes , we studied halides, haloalkenes and haloarenes. Alcohol, Phenol, and Ether are groups of organic compounds. These compounds have enormous applications in enterprises for local purposes. At the point when hydroxyl (- OH) group bonds with saturated carbon atom results are Alcohol. What’s more, dehydration of alcohol structures Ether. Monohydric, Dihydric, and Trihydric are three sorts of alcohols, given the hydroxyl gathering. In this part, we will discuss the sorts of alcohol, ether, and phenol. We will take a look at their classification and spread a couple of models.
Alcohol, Phenol, and Ether
These three are classes of organic compounds having a wide utilization in an expansive scope of ventures just as for residential purposes. Yet, what right?
Alcohol is the item we get when a saturated carbon atom bonds to a hydroxyl (- OH) gathering.
Phenol is the thing that we get when the – OH group replaces the hydrogen atom in benzene.
Ether is the item that we get when an oxygen atom bonds to two alkyl or aryl gatherings.
Quick revision notes
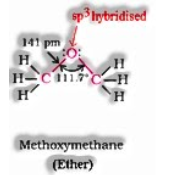
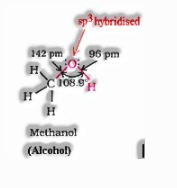
Structure of alcohols:
Preparation of alcohols:
a) From alkene
b) From esters
![]()
c) From aldehydes and ketones

d) From carboxylic acids
![]()
Structure of phenols:
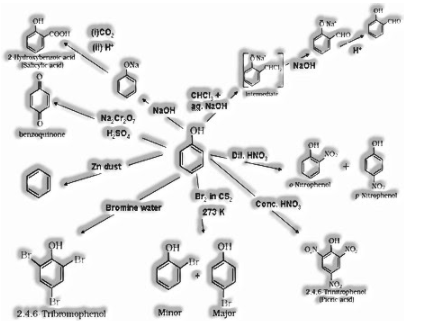
Preparation of phenols:
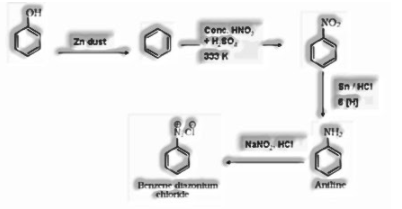
a) From benzene
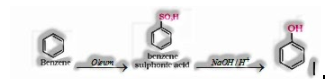
b) From chlorobenzene

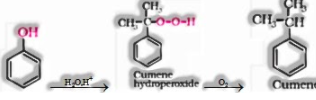
c) From cumene
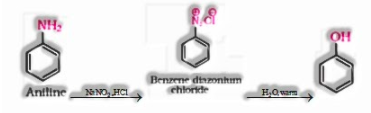
d) From aniline
Physical properties of alcohols and phenols:
a) Boiling points:
Boiling points of alcohols and phenols are higher in contrast with different classes of compounds. This is on the grounds that the – OH group in alcohols and phenols is engaged with intermolecular hydrogen bonding.
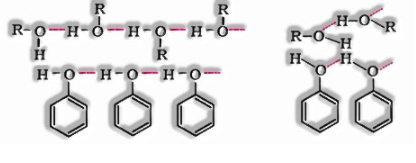
The boiling points of alcohols and phenols increment with increment in the number of carbon atoms. This is a direct result of the increment in van der Waals powers with increment in the surface region.
In alcohols, the boiling points decline with an increment of stretching in the carbon chain. This is a direct result of diminishing in van der Waals powers with a decline in the surface area.
b) Solubility:
The solubility of alcohols and phenols are dissolvable in water because of their capacity to frame hydrogen bonds with water molecules. The solubility of alcohols diminishes with increment in the size of alkyl/aryl (hydrophobic) gatherings.
Chemical properties of alcohols:
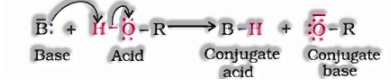
Reactions including cleavage of O–H bond: Alcohols respond as nucleophiles:
a) Reaction with metals
![]()
b) Esterification reaction
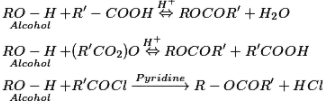
Reactions of alcohols including cleavage of carbon-oxygen (C–O) bond:
a) Reaction with hydrogen halides
![]()
b) Reaction with phosphorus trihalides
![]()
c) Dehydration reaction

d). Oxidation reaction
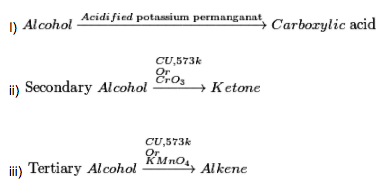
Chemical properties of phenols:
Reactions including cleavage of O–H bond: Alcohols respond as nucleophiles:
a) Reaction with metals
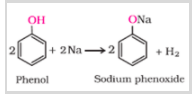
b) Esterification reaction
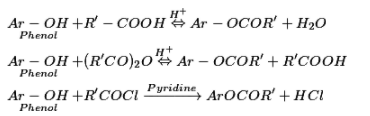
Other chemical reactions of phenols:
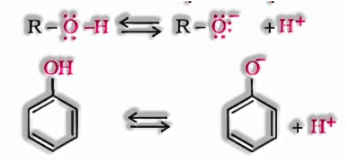
Acidic nature of phenol and alcohol:
a). Phenol > H2O (water) > Primary alcohol > Secondary alcohol > Tertiary alcohol.
The acidic character of alcohols is because of the polar nature of the O–H bond. Alkyl bunch is an electron-delivering bunch (– CH3, – C2H5) or it has electron delivering inductive impact (+I affect). Due to +I impact of alkyl gatherings, the electron thickness on oxygen increments. This declines the polarity of the O-H bond. Subsequently, the acid quality reduces.
b) Phenol is more acidic than alcohol:
In phenol, the hydroxyl bunch is straightforwardly appended to the sp2hybridised carbon of benzene ring which goes about as an electron pulling back gathering though, in alcohols, the hydroxyl bunch is joined to the alkyl bunch which have electron delivering inductive impact. In phenol, the hydroxyl bunch is straightforwardly joined to the sp2hybridised carbon of benzene ring while in alcohols, the hydroxyl bunch is appended to the sp3hybridised carbon of the alkyl gathering. The sp2hybridised carbon has a higher electronegativity than sp3hybridised carbon. In this way, the polarity of the O–H bond of phenols is higher than those of alcohols. Thus, the ionization of phenols is greater than that of alcohols.
The ionization of alcohol and a phenol happens as follows:
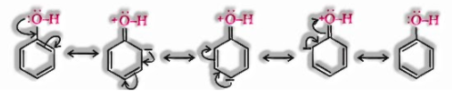
In alkoxide ion, the negative charge is restrained on oxygen while in phenoxide ion, the charge is delocalised.
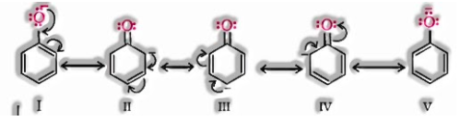
The delocalisation of negative charge shapes phenoxide ion more steadily and favours the ionization of phenol. In spite of the fact that there is likewise charge delocalisation in phenol, its resonance structures have charge separation because of which the phenol molecule is less steady than phenoxide ion.
c) In substituted phenols
the nearness of electron pulling back gatherings, for example, nitro group upgrades the acidic quality of phenol. Then again, electron delivering gatherings, for example, alkyl gatherings, by and large, diminishes the acid quality. It is on the grounds that electron pulling back gatherings lead to powerful delocalisation of negative charge in phenoxide ion.
Separate between organic compounds:
a) Alcohols and phenols
Phenol on reaction with nonpartisan FeCl3 gives purple shading while alcohols don’t give purple shading.
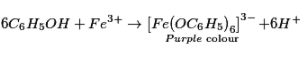
b) Primary, secondary and tertiary alcohols
Lucas reagent test:
On the off chance that it is a primary alcohol, no turbidity shows up at room temperature. Turbidity shows up just on warming. On the off chance that it is a secondary alcohol, turbidity shows up in a short time. In the event that it is a tertiary alcohol, turbidity shows up right away.
c) Methanol and ethanol
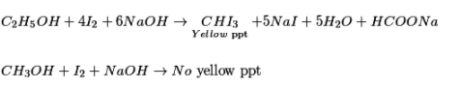
Iodoform test: Ethanol when responded with (I2 and NaOH) or NaOI gives yellow ppt of iodoform since it has the nearness of CH3-CH (OH)- gathering.
Structure of ethers:
Preparation of ethers:
a) From alcohols
![]()
b) From alkyl halide and sodium alkoxide
![]()
Here, the alkyl halide ought to be primary and alkoxide ought to be tertiary. If there should arise an occurrence of sweet-smelling ether, the fragrant part ought to be with phenoxide ion.
Physical properties of ethers
a) Miscibility: Miscibility of ethers with water matches alcohols of the equivalent molecular mass. This is because of the way that alcohols, oxygen of ether can likewise frame hydrogen bonds with a water molecule.
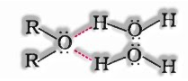
b) Boiling points:
Ethers have a lot of lower boiling points than alcohols. This is because of the nearness of hydrogen bonding in alcohols. Hydrogen bonding is missing in others.
Chemical properties of ethers:
a) Cleavage of C–O bond in ethers:
R-O-R’ + HX → R-X + R’OH
Excess
The request for reactivity of hydrogen halides is as per the following: HI >HBr>HCl
Alkyl halide shaped is consistently the lower alkyl gathering. However, in the event that a tertiary alkyl bunch is available, the alkyl halide is consistently tertiary. If there should arise an occurrence of phenolic ethers, the cleavage happens with the formation of phenol and alkyl halide.
b) Electrophilic substitution reaction in fragrant ethers:
The electrophilic substitution reaction of fragrant ether includes the accompanying reaction:
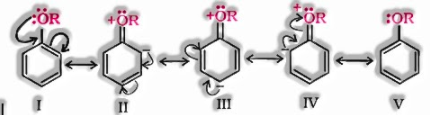
Other conversion reactions:
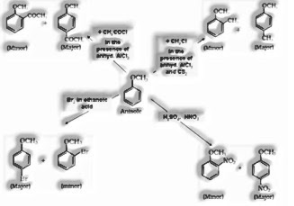
a) Phenol to salicylaldehyde
b) Phenol to benzene diazonium chloride
Questions
Q: Pen down the physical properties of phenols.
Ans: Phenol is a dull, harmful, corrosive, needle-formed strong. It liquifies because of high hygroscopic nature.
Phenol is less dissolvable in water, however, it breaks down appropriately in organic solvents.
Most straightforward phenols, on account of hydrogen bonding, have very high boiling points.
o-nitrophenol is unstable and furthermore less dissolvable in water due to intramolecular hydrogen bonding