States of Matter: Class 11 Chemistry NCERT Chapter 5
Key Features of NCERT Material for Class 11 Chemistry Chapter 5 – States of Matter
In the previous chapter: Chemical Bonding and Molecular Structure, you must have learnt about the different bonds that takes place between elements and how these bonds are necessary in the formation of a compound. In chapter 5 of NCERT Chemistry Class 11 Textbook: States of Matter, you will learn about knowing how the state of matter is done by the competition between intermolecular interactions and thermal energy.
The matter is a substance that possesses mass and occupies space. Chemistry studies it, and this study has various aspects also. Chemistry studies it, and this study has various aspects in it. One of the significant elements is its classification relying upon its chemical composition into three fundamental categories, such as elements, compounds, and mixtures. Which further classified based on its characteristic properties into metals, non-metals, and metalloids. It may very well be in liquid, gaseous, solid, or plasma state.
Will Matter Change Its Shape?
The answer is ‘Yes.’ It can change its shape, size, and volume. For example, the water turns into ice after freezing, here the type of water converts from the liquid state into the solid-state; the matter itself doesn’t change; however, it transforms its shape. The components of water continue as before, just the shape changes. Evaporation varies the type of water from liquid state to gaseous state.
Its physical state depends on physical conditions such as temperature, atmosphere, dampness, and so on. Usually, to change the state of liquids into a solid-state, one reduces the temperature. For the most part, liquids are less dense than solids because their molecules have little or no space in the middle of them. The freezing process distributes the molecules into a smaller space.
Quick revision notes
-
Intermolecular Forces
Intermolecular forces are the fundamental forces of attraction and repulsion between connecting particles have permanent dipole moments. This interaction is more durable than the London forces yet is more vulnerable than ion-ion communication because just fractional charges are included.
The alluring forces decrease with the increasing distance between the dipoles. The interaction energy is directly proportional to 1/r6, where r is the distance between polar molecules.
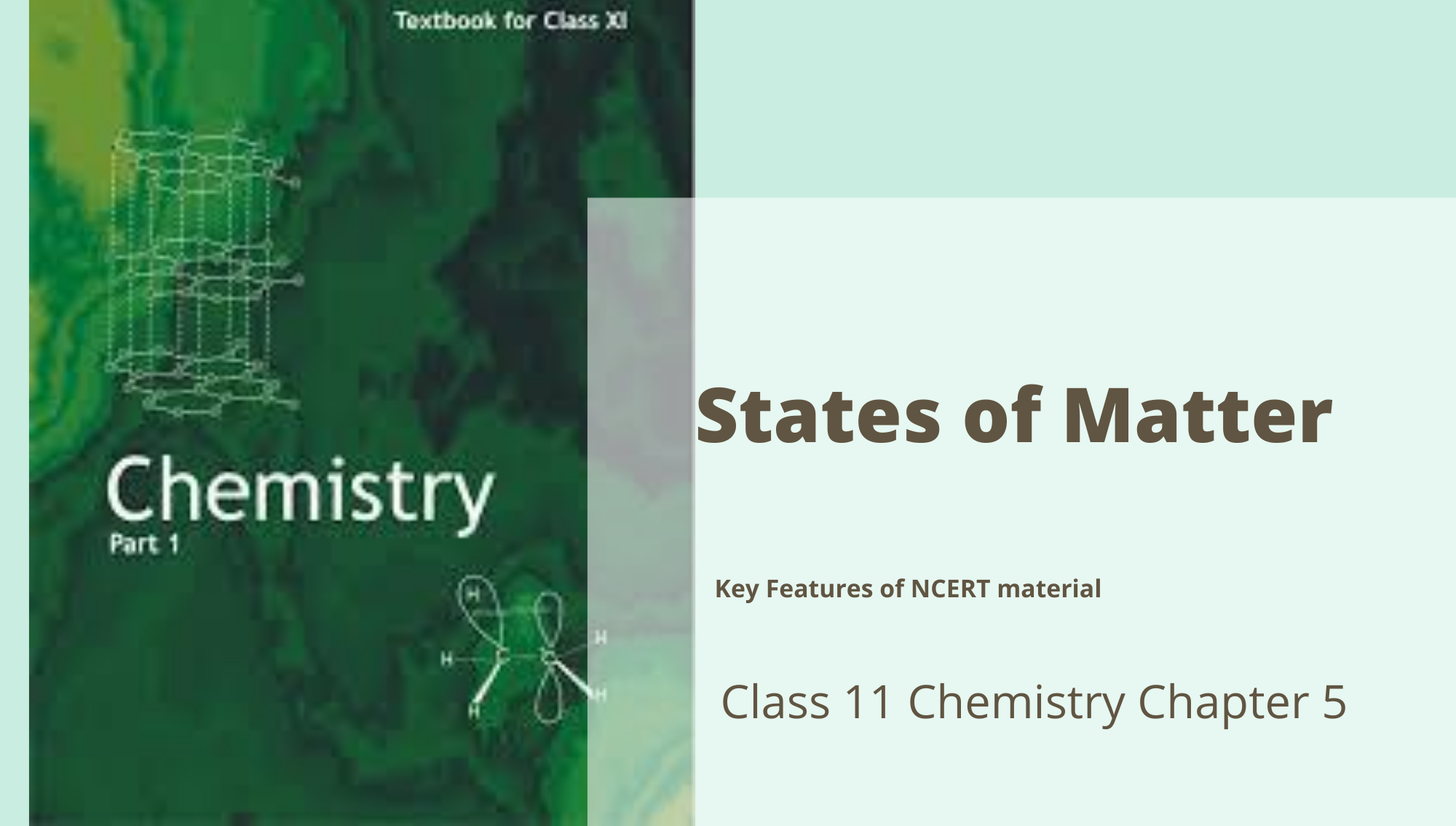
Ion-Dipole Interaction: This is the power of attraction that exists between the ions (cations or anions) and polar molecules. The atom is pulled in towards the oppositely charged finish of dipolar molecules.
The strength of attraction depends primarily upon the charge and size of the ion and the dipole moment and the size of the polar molecule.
For example, Solubility of basic salt (NaCl) in water.
-
Ion-prompted Dipolar Interactions
In this kind of interaction, the permanent dipole of the polar molecule induces dipole on the electrically neutral molecule by misshaping its electronic cloud. The interaction energy is directly proportional to 1/r6, where r is the distance between two molecules.
-
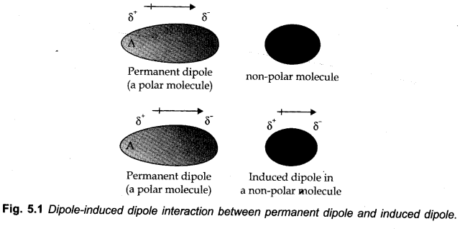
London Forces or Dispersion Forces
As we realize that in non-polar molecules, there is no dipole moment because of their electronics. The charge cloud is symmetrically distributed. However, it is accepted that at any instant of time, the electron haze of the molecule might be distorted so that an instantaneous dipole or momentary dipole is delivered in which one piece of the molecule is slightly more negative than the other part. This momentary dipole induces dipoles in the neighboring molecules. Thus, the power of attraction exists among them and is actually the same as between permanent dipoles. This power of attraction is known as London forces or Dispersion forces. These forces are always alluring. The interaction energy is basically inversely proportional to the sixth intensity of the distance between two interfacing particles (for example, 1/r6 where r is the distance between two particles).
This can be shown by fig. given beneath.
Hydrogen bonding
When hydrogen iota is joined to the profoundly electronegative element by covalent bond, electrons are shifted towards the more electronegative particle. Thus a halfway positive charge develops on the hydrogen particle. Presently, the positively charged hydrogen particle of one molecule may pull in the adversely charged iota of some other molecule, and the two molecules can be connected together through a feeble power of attraction.
![]()
Thermal Energy
The energy arising because of the molecular motion of the body is known as warm energy. Since the motion of the molecules is legitimately identified with kinetic energy and kinetic energy is straightforwardly proportional to the temperature.
-
The Gaseous State
Physical Properties of Gaseous State
(I) ases have no unmistakable volume, and they don’t have specific shape,
(ii) Gases blend wholly and equitably in all proportions with no mechanical guide.
(iii) Their density is a lot lower than solids and liquids. :
(iv) They are profoundly compressible and apply pressure similarly every which way.
-
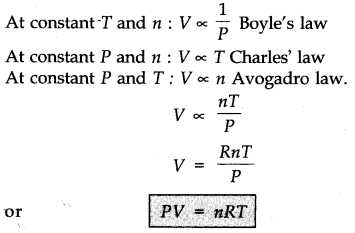
Boyle’s Law (Pressure-Volume Relationship)
At a constant given temperature, the volume of a mass of gas is inversely proportional to its pressure

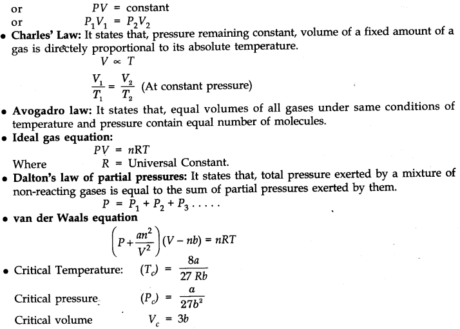
Charles’ law
At constant pressure, the volume of a given mass of a gas is legitimately proportional to its absolute temperature.

Gay Lussac’s Law (Pressure-Temperature Relationship)
At a constant given pressure, the volume of a given mass of a gas is legitimately proportional to its absolute temperature.

Avogadro Law (Volume-Amount Relationship)
Avogadro’s law states that equivalent volumes of all gases under the same conditions of temperature and pressure contain the equivalent number of molecules.
V α n
Where n is the quantity of moles of the gas.
Avogadro constant: The quantity of molecules in a single mole of a gas
= 6.022 x 1023
Ideal Gas
A gas that follows all the laws, including Boyle’s law, Charles’ law, and Avogadro law, is called an ideal gas.

Real gases adhere to these laws just under certain conditions when forces of interaction are negligible for all intents and purposes.
Ideal Gas Equation
This is the joined gas equation of three laws and is known as an ideal gas equation.
Dalton’s Law of Partial Pressure
At the point when at least two non-receptive gases are enclosed in a vessel, the absolute pressure applied by the gaseous blend is equivalent to the sum of the fractional pressure of individual gases.
Let P1, P2, and P3 be three pressure of non-responsive gases A, B, and C. At the point when enclosed separately in the same volume and under the same condition.
total = P1+ P2 + P3
Where PTotal = P is the absolute pressure applied by the blend of gases.
Aqueous Tension
The pressure of non-responding gases is commonly collected over water and consequently are moist. The pressure of dry gas can be determined by subtracting the fume pressure of water from the complete pressure of the moist gas.
P2Dry gas = PTotal – Aqueous Tension
Partial Pressure in terms of Mole Fraction
Let at the given temperature T, three gases enclosed in volume V, apply fractional pressure P1, P2 and P3 respectively, at that point
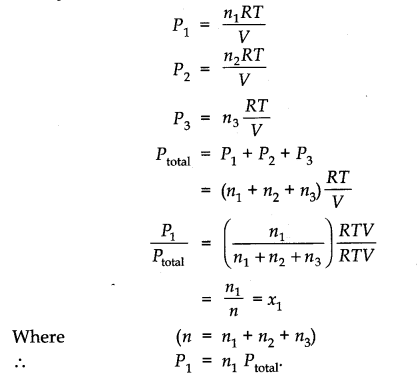
Kinetic Molecular Theory of Gases
(I) Gases consist of a huge number of small indistinguishable particles (atoms or molecules),
(ii) the Actual volume involved by the gas molecule is negligible compared to discharge space between them.
(iii) Gases can consume all the space available to them. This means they don’t have any power of attraction as such between their particles.
(iv) Particles of a gas are always in the constant arbitrary motion.
(v) When the gas particles are in arbitrary motion, the pressure is applied by the gas because of the collision of the particles with the walls of the compartment.
(vi) The collision of the gas molecules is perfectly elastic. This clearly means there is no loss in energy after the collision. There might be just trade of energy between impacting molecules.
(vii) At a specific temperature distribution of speed between gaseous particles remains constant.
(viii) The average kinetic energy of the gaseous molecule is straightforwardly proportional to the absolute temperature.
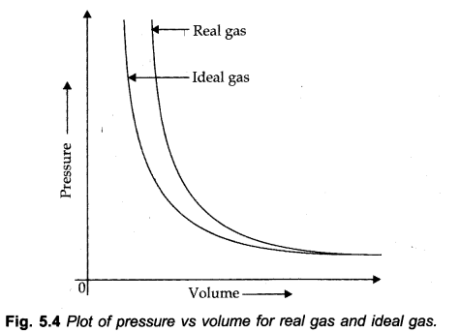
Deviation From Ideal Gas Behavior
Real Gas: A gas which does not follow ideal gas conduct under all conditions of temperature and pressure, is called real gas.
Deviation with respect to pressure can be studied by plotting pressure Vs. volume bend at a given temperature. (Boyle’s law)
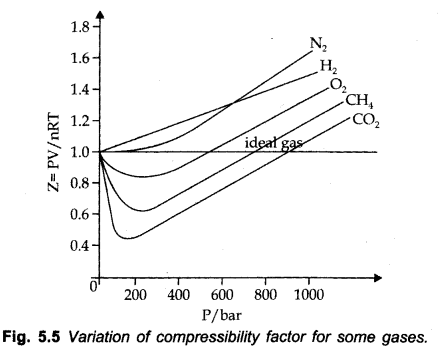
Compressibility factor (Z )
Deviation from ideal conduct can be measured in terms of compressibility factor, Z.

Van der Waals Equation
![]()
Where V is a constant for molecular attraction while ‘V is a constant for molecular volume.
(a) There is no power of attraction between the molecules of a gas.
(b) The volume involved by the gas molecule is negligible in comparison to the total volume of the gas.
Over two assumptions of the kinetic hypothesis of gas were seen as off-base at extremely high pressure and low temperature.
Liquefaction of Gases
Liquefaction of gases can be accomplished either by bringing down the temperature or increasing the gas’s pressure simultaneously.
Thomas Andrews plotted isotherms of C02 at various temperatures shown in the figure.
.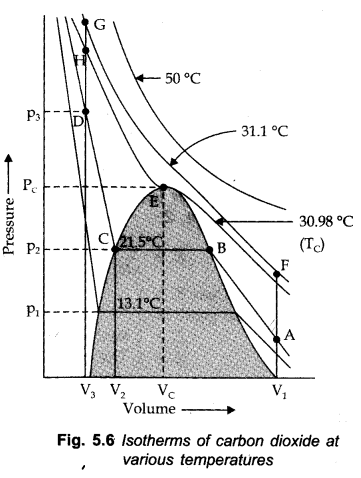
Critical Temperature (Tc)
It is characterized as that temperature above which a gas can’t be liquified anyway high pressure might be applied on the gas.
Tc = 8a/27bR
(Where an and b are van der Waals constants)
Critical Pressure (Pc)
It is characterized as that temperature above which a gas can’t be liquified anyway; high pressure might be applied to the gas.
Tc = 8a/27bR
(Where an and b are van der Waals constants)
Critical Pressure (Pc): It is the pressure required to Liquify the gas at the critical temperature.
Pc = a/27b2
The volume involved by one mole of the gas at the critical temperature and the critical pressure is called the critical volume (Vc).
For Example. For C02 to Liquify.
Tc = 30.98°C
Pc = 73,9 atm.
Vc = 95-6 cm3/mole
All the three are collectively called critical constants
Liquid State
Characteristics of Liquid State
(I) In liquid, intermolecular forces are strong in comparison to the gas.
(ii) They have distinct volumes, however irregular shapes, or we can say that they can take the shape of the holder.
(iii) Molecules of liquids are held together by appealing intermolecular forces.
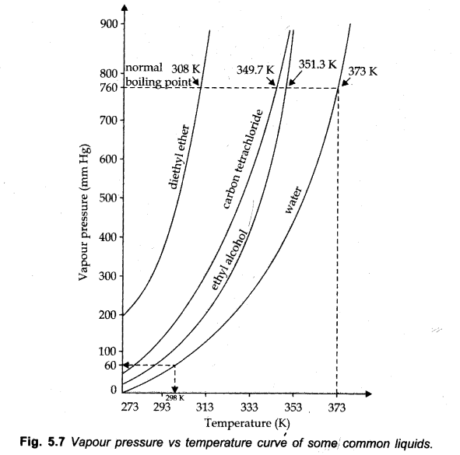
Vapour Pressure: The pressure applied by the fume of a liquid, at a specific temperature in a state of dynamic equilibrium, is called the fume pressure of that liquid at that temperature.
Vapour Pressure depends on two factors:
(i) Nature of Liquid (ii) Temperature
-
Surface Tension
It is characterized as the power acting per unit length perpendicular to the line drawn on the surface of liquid. S.I. unit of Surface Tension = Nm-1
Surface Tension decreases with increase in temperature, because power acting per unit length decreases because of increase in kinetic energy of molecules.
-
Viscosity
It is characterized as the internal resistance to the stream possessed by a liquid.
The liquids that stream slowly have extremely high internal resistance because of strong intermolecular forces and thus are said to be more viscous.
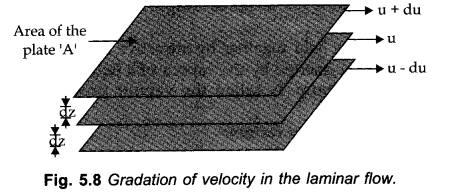
When liquid flows, the layer quickly underneath it tries to impede its stream while the one above tries to accelerate.
Thus, power is required to keep up the progression of layers.

Effect of Temp, on Viscosity
Viscosity of liquids decreases as the temperature rises. At high temperatures, molecules have high kinetic energy and can conquer the intermolecular forces to slip past each other.
-
Boyle’s Law
It states that under isothermal conditions, the tension of a given mass of a gas is inversely proportional to its volume.
![]()
Questions
Q1. Which of the accompanying process converts solid type of matter to a gaseous structure?
- Sublimation
- Condensation
- Fusion
- Deposition
Ans: Sublimation. Sublimation is a phase transition of a matter that changes it straightforwardly from the solid state to liquid state skipping the liquid transition phase.

