Acids Bases and Salts: Class 7 Science NCERT Chapter 5
Key Features of NCERT Material for Class 7 Science Chapter 5 – Acids,...

Introducing you all to the 12th Chemistry, In the first chapter: The Solid State, you will learn about solid and its properties like the properties of solids depend upon the idea of constituent particles.
(The Solid State: Class 12)
This bit of substance manages a strong state. As a matter of first importance, we think about fluids and gases as liquids. This is a direct result of their capacity to stream. Also, this smoothness is conceivable in light of the fact that their particles are permitted to move about. Also, the places of constituent particles in solids are fixed, and they can simply sway. Thus, this outcomes in unbending nature in solids.
Besides, the properties likewise depend upon the coupling powers which work between the constituent particles. In addition, the connection between’s the properties and structure encourages the revelation of new strong materials. Generally imperative, various game plans of particles can be made with the strong state. Along these lines, strong state notes will come helpful while considering this subject.
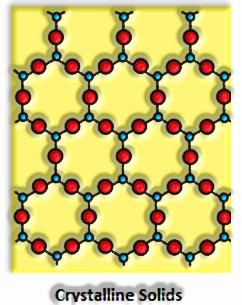
Strong: Solid is a condition of issue where the comprising particles are arranged minimalistically. The constituent particles could be iotas, atoms, or particles.
They have distinct mass, volume, and shape.
They are compressible and inflexible.
Intermolecular separations are very short, and consequently the intermolecular powers are solid.
Their constituent particles have a fixed position. Sand can simply waver about their mean positions.
(The Solid State: Class 12)
They have an unequivocal mathematical shape.
They have a long-run demand.
They have a sharp liquefying point.
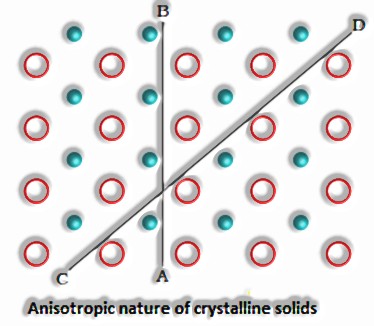
They are anisotropic; for instance, their physical properties show various qualities when estimated alongside various bearings in a similar precious stone.
They have unmistakable warmth of combination.
They are called genuine solids.
Right when cutting with a sharp-edged apparatus, they split into two pieces, and the as of late created surfaces are plain and smooth.
(The Solid State: Class 12)
The unmistakable glasslike types of a substance are known as polymorphic structures or polymorphs, for example, graphite and gem.
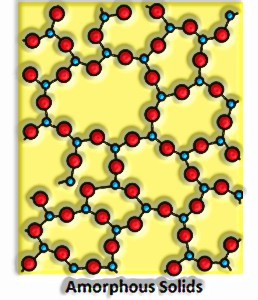
They have an unpredictable shape.
They have a short-run demand.
They constantly mellow over the organization of temperature.
They are isotropic in nature. For instance, their physical properties are the equivalent all over.
Exactly when cut with a sharp-edged instrument, they split into two pieces with unpredictable surfaces.
They don’t have a particular warmth of combination.
They are called pseudo solids or supercooled fluids. This is on the grounds that they will in general stream, however gradually.
Sub-atomic Solids
Particles: Molecules
Constituent Particles: IonsB. Ionic Solids
Holding/Attractive Forces: Coulombic or Electrostatic
Electrical Conductivity: Insulators in a strong state however leads in the liquid state and watery arrangements
Physical Nature: Hard however weak
Softening Point: High MP
Models: ZnS, MgO, and so forth
Including Particles: Positive particles in an ocean of delocalized electrons
Compound Bonding: Metallic holding
Conductivity Conductors in the strong state just as in liquid state
Physical Nature: Hard however moldable and bendable
Softening Point: Fairly high
Models: Fe, Cu, Ag, Mg
Covalent or NetworkSolids
Constituent Particles: Atoms
Holding/Attractive Forces: Covalent holding
Electrical Conductivity: Conductors in a strong state just as in liquid state
Physical Nature: Hard however moldable and bendable
Softening Point: Fairly high
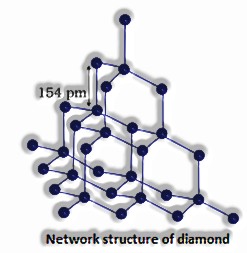
Models: , (quartz), SiC, C (valuable stone), C(graphite
Gem lattice: A customary masterminded course of action of constituent particles in three measurements is known as a precious stone lattice.
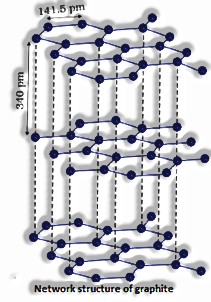
(The Solid State: Class 12)
Lattice focuses: the fixed situations on which the constituent particles present are called lattice focuses or lattice locales. At the point when rehashed time and in 3 measurements, a social occasion of lattice focuses gives the total precious stone lattice.
Unit cell: It is portrayed as the littlest rehashing unit in space lattice, which, when rehashed time and, makes the total lattice. The precious stone can contain a boundless number of unit cells in it.
Variables which portray a unit cell: Dimensions of every unit cell along the three edges, a, b, and c: these edges could be commonly opposite.
The tendency of the edges to one another: the edge between the edges means this, and respectively.is the point between the edges b and c, is the edge between the edges an and c and is the edge among an and b.
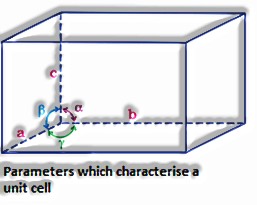
for, Cubic: ===90° ,a=b=c
for, Tetragonal: ===90° ; a=bc
for, Orthorhombic: ===90°; ABC
for, Monoclinic: ==90°,90°; abc
for, Hexagonal: ==90°,=120°; a=bc
for, Rhombohedral or three-sided: = =90°;a=b=c
for, Triclinic: 90°;abc
Crude or straightforward unit cells have particles present precisely at its corners.
Centered unit cells are those unit cells where in any event one constituent molecule is present at positions notwithstanding those present at the corners.
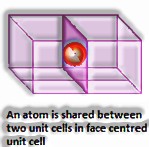
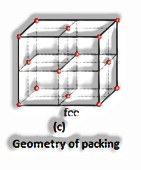
A face-centered unit cell comprises of one constituent molecule present at the focal point of each face and those present at the corners.
Body-centered unit cell: It comprises of one constituent molecule present at its body place notwithstanding the particles present in corners.
End centered unit cell: It includes one constituent present at the focal point of any of the two inverse sides and having particles at the corners too.
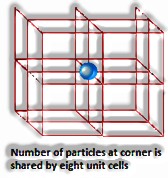
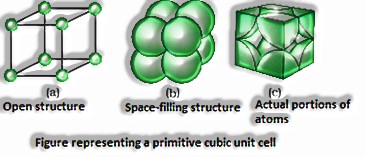
(The Solid State: Class 12)
A face-centered unit cell comprises of one constituent molecule present at the focal point of each face and those present at the corners.

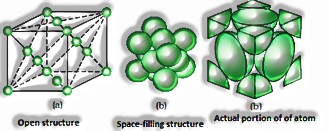

Body-centered unit cell: It comprises of one constituent molecule present at its body place notwithstanding the particles present in corners.



End centered unit cell: It contains one constituent present at the focal point of any of the two inverse sides and having particles at the corners too.
Crude unit cells have 1atom.
Face centered unit cell has three iotas.
Body-centered unit cells have 2atoms.
Coordination number: Coordination number is the amount of closest neighbors of a molecule.
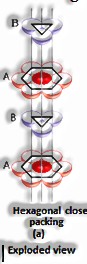
Close pressed structures:


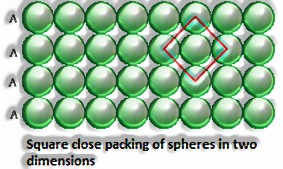
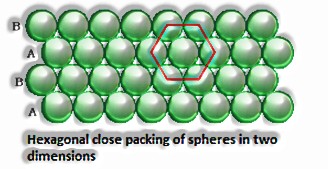
Close squeezing in two measurements: It is produced by stacking the lines of personal stuffed circles in two different ways: I) Square close squeezing and ii) Hexagonal close squeezing.
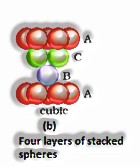
Close squeezing in three measurements: They can be gotten by stacking the two-dimensional layers one over the other. It tends to be accomplished in two different ways: I) Square close stuffed layers and ii) Hexagonal close squeezed layers.
Square close squeezing: Here, the circles of the second line item put precisely over those of the primary line. Along these lines the circles are balanced on a level plane just as vertically. The course of action is an AAA type. Four is the coordination number,


Three-dimensional pressing got from 2D square close-stuffed layers: In this, the circles of the upper layer are set decisively over the primary layer, with the end goal that the circles of the layers are accurately balanced on a level plane and vertically. It has an AAAA type design. The lattice is a basic cubic lattice.
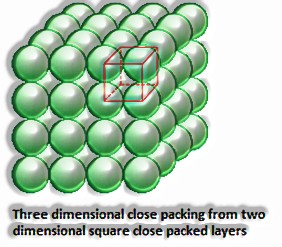

(The Solid State: Class 12)
Three-dimensional close pressings from two dimensional hexagonal tight, stuffed layers: There are two stages required as:
Putting these bond layer over the main layer
Putting the third layer during the time layer
There emerge two prospects:
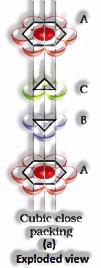

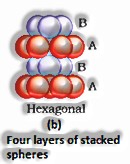

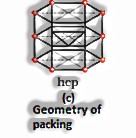





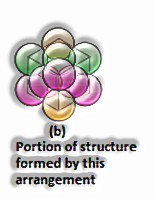

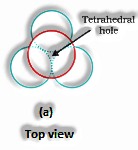

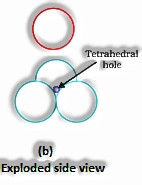

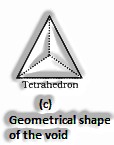

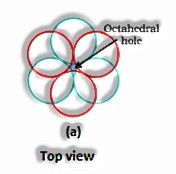

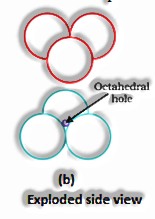

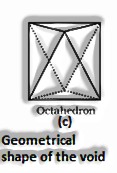

(The Solid State: Class 12)
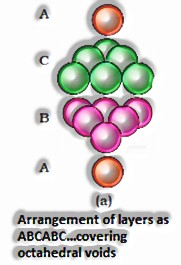
If the aggregate sum of close squeezed particles = n
All out Number of particles present in octahedral voids = n
At that point, the amount of particles present in tetrahedral voids = 2n
Squeezing profitability: It is the level of all out space required by constituent particles (iotas, atoms, or particles).
Squeezing efficiency for face-centered unit cell =74%
Squeezing efficiency for body-centered cubic unit cell =68%
Squeezing efficiency for the cubic unit cell =52.4%
Range proportion in an octahedral void: For a particle to have an octahedral void, its span ought to be 0.414 occasions more than the sweep of the circle
Span proportion for tetrahedral void: For an iota to have a tetrahedral void, its range must be 0.225 occasions the sweep of the circle.
The thickness of a unit cell is equivalent to the thickness of the substance. Connection between span of constituent particle(r) and edge length(a):
Basic cubic unit cell: a=2r
Face centered unit cell: a=
Body centered unit cell: a=
Volume of a unit cell=(edge length)3=
Basic cubic unit cell: Volume=
Face centered unit cell: Volume=
Body centered unit cell: Volume=
Number of particles in a unit cell(z):
Basic cubic unit cell: z = 1
Face centered unit cell: z = 4
Body centered unit cell: z = 2
Thickness of unit cell =
Precious stone imperfections are fundamentally anomalies in the game plan of constituent particles.
Point deserts Point surrenders are the inconsistencies or deviations from perfect course of action around a point or an iota in a translucent substance.
Line deserts Line surrenders are the inconsistencies or deviations from the perfect association in whole lines of lattice focuses.
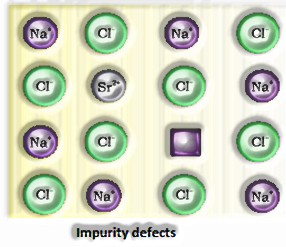

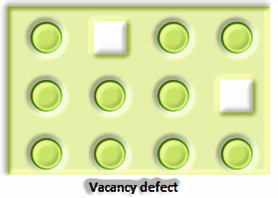

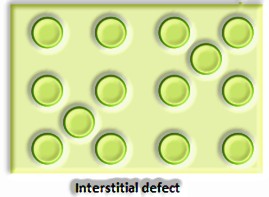

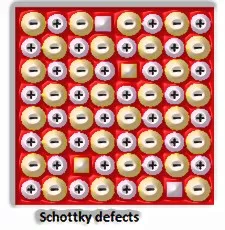



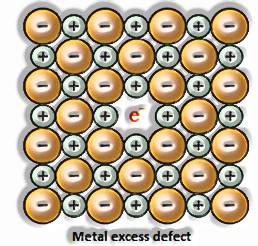

a) Anionic opportunities: A compound may have an additional metal molecule if the negative molecule is missing from its lattice site. This vacant lattice site is known as an opening. To keep up electrical impartiality, this site is included by an electron. The gap required by a particle is called f-focus or Farbenz enter focus. The F-focus is answerable for the shade of the compound.
b) Presence of extractions: A compound is said to have additional cations if a cation is present in the interstitial site. An electron is present in the interstitial site to keep up electrical impartiality.
(The Solid State: Class 12)
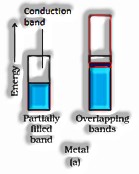
a) Conductors: The solids with conductivities going between to are called conductors.
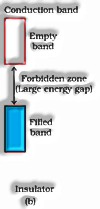
b) Insulators: These are the solids with uncommonly low conductivities stretching out between to. to.
c) Semi-conductors: These are the solids with high conductivities in the middle range. to.

b) In separators, the electrons can’t energize the conduction band.

c) For the situation of semiconductors, the distinction in groups is little. Accordingly, a few particles may jump to the conduction band and give some conductivity. The electrical conductivity of semiconductors increments with the ascent in temperature.


a) Intrinsic semiconductors: These are those semiconductors wherein the no-no opening is little. Simply a few electrons may bounce to the conduction band and give some conductivity. They have low electrical conductivity. Model: Silicon, germanium.
b) Extrinsic semiconductors: When a suitable contamination is added to an inborn semiconductor, it is called outside semiconductors. Their electrical conductivity is high.
Doping: The way toward including a fitting measure of appropriate polluting influence to build the conductivity of semiconductors is known as doping.
a) The n-type semiconductors: They are confined when silicon is doped with electron-rich debasement like social occasion 15 components. The expansion in conductivity is a result of the contrarily charged electrons.
b) The p-type semiconductors are encircled when silicon is doped with an electron-insufficient polluting influence, such as social affair 13 components. The expansion in conductivity is a result of the decidedly charged gaps.
Doping: The way toward including a fitting measure of reasonable debasement to expand the conductivity of semiconductors is known as doping.
a) The n-type semiconductors: They are confined when silicon is doped with electron-rich polluting influence like get-together 15 components. The expansion in conductivity is a result of the adversely charged electrons.
b) The p-type semiconductors are confined when silicon is doped with an electron-lacking contamination, such as social event 13 components. The expansion in conductivity is a result of the decidedly charged openings.
Diode: It is a mix of n-type and p-type semiconductors and is utilized as a rectifier.
Semiconductors are built by sandwiching a layer of one sort of semiconductor between two layers of the other semiconductor. The npn and pnp sort of semiconductors are utilized to escalate radio or sound signs.
The 12-16 exacerbates: These mixes are formed by the mix of social event 12 and get-together 16 mixes. They have an ordinary valency of 4.Examples – ZnS, CdS, CdSe, and HgTe.
The 13-15 exacerbates: These mixes are formed by the blend of social affair 13 and get-together 15 mixes. They have a typical valency of 4.Examples – InSb, AlP, and GaAs.
Every substance has some attractive properties related with it. The origin of these properties lies in the electrons.
Each electron in a particle carries on like a magnet. Its attractive second starts from two kinds of movements:
(I) its orbital movement around the core and
(ii) it’s turn around its hub.
Paramagnetic substances: These are those substances which are sadly pulled in by the attractive field. It is a direct result of the nearness of at any rate one unpaired electron.
Diamagnetic substances: An attractive field weakly repulses diamagnetic components. Diamagnetism is appeared by those substances where all the electrons are matched, and there are no unpaired electrons.
Ferromagnetic substances: These are substances that are pulled in each effectively by an attractive field.
Hostile to ferromagnetic substances have a proportional number of equal and antiparallel attractive dipoles bringing about a zero net dipole second.
Ferromagnetic substances: They have a conflicting number of equal and antiparallel attractive dipoles bringing about an at dipole second.
Q: A compound formed by components An and B takes shape in the cubic structure where An iotas are at the edges of a strong shape, and B particles are at the face habitats. The formula of the compound is
AB3
Stomach muscle
A3B
A3B3
Sol: The right answer is “A.” The count will be as per the following.
Number of An iotas = 1/8 × 8 = 1
Number of B iotas = 1/2 × 6 = 3
(The Solid State: Class 12)
0 responses on "The Solid State: Class 12 Chemistry NCERT Chapter 1"