Surface Chemistry: Class 12 Chemistry NCERT Chapter 5

Characteristics of NCERT Class 12 Chemistry Chapter 5 – Surface Chemistry
In the last chapter 4, you learned about Chemical Kinetics. In this chapter, you will learn about Surface Chemistry. You probably wondered how the particles in particular kinds of solutions stay suspended constantly without settling down. What precisely occurs at the interface between different phases? The part of chemistry worried about the cycles occurring at interfaces between phases, particularly that among liquid and gas is known as surface chemistry. Surfaces assume a functioning job in catalysis, colloid arrangement, electrode reactions, chromatography, etc. Let us investigate more about surface chemistry and its shifted applications in different fields. You probably wondered how the particles in specific kinds of solutions stay suspended without settling down. What occurs at the interface between phases? The part of chemistry worried about the cycles occurring at interfaces between phases, particularly that among liquid and gas is known as surface chemistry.
(Surface Chemistry: Class 12 )
Quick Revision Notes
Adsorption:
The aggregation of atomic species at the surface, as opposed to in the greater part of a substantial or liquid, is named adsorption.
It is a surface marvel.
The centralization of adsorbate increments just at the outside of the adsorbent.
Adsorbate: It is the substance that is being adsorbed on the outside of another substance.
Adsorbent: It is the substance present in mass, on the outside of which adsorption is occurring.
Desorption: It is the way toward eliminating an adsorbed substance from a surface on which it is adsorbed.
Retention:
It is a marvel wherein a substance is consistently distributed all through the main part of the strong.
It is a mass wonder.
The focus is uniform all through the heft of strong.
Sorption: When adsorption and ingestion occur at the same time, it is called sorption.
Enthalpy or warmth of adsorption: Since, adsorption happens with discharge in vitality, i.e., it is exothermic. The change in enthalpy for the adsorption of one mole of an adsorbate is called enthalpy or warmth of adsorption.
Kinds of adsorption: There are different sorts of adsorption precisely,
-
- Physical adsorption
- Synthetic adsorption
- Physical adsorption
(I) If the adsorbate is hung on a surface of adsorbent by powerless van der Waals’ powers, the adsorption is called physical adsorption or physisorption.
(ii) It is vague.
(iii) It is reversible.
(iv) The gas measure relies on the nature of gas, i.e., virtually liquefiable gases like NH3, CO2, gas adsorbed to a more noteworthy degree than H2 and He. Higher the basic temperature of gas more will be the degree of adsorption.
(v) The degree of adsorption increments with increment in the surface territory, such as porous and finely divided metals, is acceptable adsorbents.
(vi) There are frail van der Waals’ powers of fascination among adsorbate and adsorbent.
(vii) It has a low enthalpy of adsorption (20 – 40 kJ mol-1).
(viii) The low temperature is good.
(ix) No calculable initiation vitality is required.
(x) It structures multimolecular layers.
(Surface Chemistry: Class 12 )
Compound adsorption or chemisorption:
(I) If the adsorbate’s powers are as solid as in compound bonds, the adsorption cycle is known as substance adsorption of chemisorption.
(ii) It is profoundly explicit.
(iii) It is irreversible.
(iv) The measure of gas adsorbed isn’t identified with the basic temperature of the gas.
(v) It additionally increments with increment in the surface region.
(vi) There is a solid power of fascination like a concoction bond.
(vii) It has enthalpy warmth of adsorption (180 – 240 kJ mol-1).
(viii) High temperature is ideal.
(ix) High enactment vitality is, in some cases, required.
(x) It structures unimolecular layers.
Variables influencing adsorption of gases on solids:
- Nature of adsorbate: Physical adsorption is vague, and in this way, every gas gets adsorbed on the outside of any strong to a lesser or more prominent degree. Be that as it may, effectively liquefiable gases like NH3, HCl, CO2, and so on which have higher basic temperatures are assimilated to a more prominent degree though H2, O2, N2, and so on. Are adsorbed to a lesser degree. The substance adsorption being exceptionally explicit, thus, a gas gets adsorbed on explicit strong just on the off chance that it goes into concoction blend with it.
- Nature of adsorbent: Metal oxides like aluminum oxide, mud, and Activated Carbon are generally utilized as adsorbents. They have their particular adsorption properties depending upon pores.
- Explicit zone of the adsorbent: The more prominent the particular territory, the more likely the adsorption. That is the reason permeable or finely divided types of adsorbents adsorb more massive amounts of the adsorbate. The pores ought to be sufficiently enormous to permit the gas molecules to enter.
- The pressure of the gas: Physical adsorption increments with increment in pressure.
Adsorption isotherm: Methods for a bend can communicate the variety in the measure of gas adsorbed by the adsorbent with pressure at a consistent temperature named adsorption isotherm.
Freundlich Adsorption isotherm: The connection betwixt ![]() and pressure of the gas at consistent temperature is known as adsorption isotherm and is given by
and pressure of the gas at consistent temperature is known as adsorption isotherm and is given by
![]()
Where x-mass of the gas adsorbed on mass m of the adsorbent and the gas at a specific temperature k and n relies on the idea of gas
The solid first increments with increment in pressure at low pressure yet get autonomous pressure at high pressure.
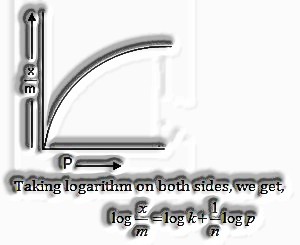
Taking logarithm on two sides, we get,
![]()
We get a straight line on the off-chance that we plot a chart between logand log P.
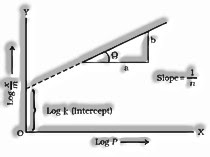
The slant of the line isand the block will be intercepted to log k.
- Impetus: These are substances that adjust the pace of a concoction reaction, and themselves remain chemically and quantitatively unaltered after the reaction, and the wonder is known as catalysis.
- Promoters: These are the substances which increment the action of impetus. Model – Mo is an advertiser, while Fe is an impetus in Haber’s Process.

Reactant harms (Inhibitors): These are the substances that decline the movement of impetus. Model – Arsenic goes about as reactant poison in corrosive sulphuric production by ‘contact measure.’
(Surface Chemistry: Class 12 )
Sorts of catalysis:
There are two sorts of catalysis in particular,
Homogeneous catalysis: When the impetus and the reactants are in a similar phase, this sort of synergist measure is known as homogeneous catalysis.
Heterogeneous catalysis: When the impetus and the reactants are in different phases, the synergist cycle is supposed to be heterogeneous.
Movement of impetus: The impetus can expand the pace of a concoction reaction.
The selectivity of impetus: Impetus can direct a reaction to yield a specific product (excluding others).
For instance: CO and H2 respond to frame different products in the nearness of different impetuses as follows:
![]()
![]()
![]()
-
- Shape – selective catalysis: The catalysis relies on the pore structure of the impetus and sub-atomic size of reactant and product molecules. Model – Zeolites are shape-selective impetuses because of their honeycomb structure.
- Enzymes: These are intricate nitrogenous organic compounds that are delivered by living plants and creatures. They are protein molecules of high atomic mass. They are biochemical impetuses.
- Steps of enzyme catalysis:
-
- (I) Binding of the enzyme to the substrate to frame an activated complex.
- (ii) Decomposition of the activated complex to shape the product.
- Qualities of enzyme catalysis:
- (I) They are exceptionally productive. 106 molecules of reactants require only one molecule of enzyme for every movement.
- (ii) They are exceptionally explicit. Model – Urease catalysis hydrolysis of urea as it were.
- (iii) They are dynamic at ideal temperatures (298 – 310 K). The pace of enzyme-catalyzed reaction gets most extreme at an unequivocal temperature called the ideal temperature.
- (iv) They are profoundly dynamic at a particular pH called ideal pH.
- (v) Enzymatic action can be expanded in the nearness of coenzymes, which can be called promoters.
- (vi) Activators are commonly metal particles Na+, Co2+ and Cu2+, and so forth. They pitifully tie to the enzyme and increment its action.
- (vii) Influence of inhibitors (poison): Enzymes can likewise be repressed or harmed by specific substances’ nearness.
- True solution:
- (I) It is homogeneous.
- (ii) The diameter of the particles is under 1 nm.
- (iii) It goes through channel paper.
- (iv) Its particles can’t be seen under a magnifying lens.
- Colloids:
-
- (I) It seems, by all accounts, to be homogeneous, however, is heterogeneous.
- (ii) The radius of the particles is two times between 1 nm to 1000 nm.
- (iii) It goes through ordinary channel paper, however not through ultra-channels.
- (iv) An incredible magnifying lens can see its particles because of the scattering of light.
- Suspension:
- (I) It is heterogeneous.
- (ii) The diameter of the particles is bigger than 1000 nm.
- (iii) It doesn’t go through channel paper.
- (iv) Its particles can be seen even with the unaided eye.
- Dispersed phase: The substance that gets added into the medium and gets completely dispersed also.
- Dispersion medium: The substance present in a larger amount is known as the dispersed medium.
- Classification of colloids on the grounds of the physical condition of the dispersed medium and phase:
|
Name |
Dispersed phase |
Dispersed medium |
Examples |
|
Solid sol |
solid |
Solid |
Coloured gemstones |
|
Sol |
Solid |
Liquid |
Paints |
|
Aerosol |
Solid |
Gas |
Smoke, dust |
|
Gel |
Liquid |
Solid |
Cheese, jellies |
|
Emulsion |
Liquid |
Liquid |
Hair cream, milk |
|
Aerosol |
Liquid |
Gas |
Mist, fog, cloud |
|
Solid sol |
Gas |
Solid |
Foam rubber, pumice stone |
|
Foam |
Gas |
Liquid |
Whipped cream |
- Classification of colloids on the basis of nature of interaction between dispersed phase and dispersion medium, the colloids are classified into two types namely,
Lyophobic sols
Lyophilic sols
- Lyophobic sols:
- I) These colloids are liquid loathing.
(ii) In these colloids, the particles of the dispersed phase have no penchant for the dispersion medium.
(iii) They are not steady.
(iv) They can be set up by blending substances directly.
(v) They need to balance out specialists for their protection.
(vi) They are irreversible sols.
Lyophilic sols:
(I) These colloids are liquid adoring.
(ii) In these colloids, the particles of the dispersed phase have an extraordinary liking for the dispersion medium.
(iii) They are steady.
(iv) They can’t be set up by blending substances directly. They are arranged distinctly through exceptional strategies.
(v) They don’t require balancing out specialists for their protection.
(vi) They are reversible sols.
Classification of colloids based on sorts of particles of the dispersed phase:
There are three sorts of colloids dependent on the kind of dispersed phase, to be specific,
Multimolecular colloids: The colloids where the colloidal particles comprise of totals of atoms or little molecules. The diameter of the colloidal molecule is under 1 nm.
Macromolecular colloids: These are the colloids where the dispersed particles are themselves huge molecules (typically polymers). Since these molecules have dimensions similar to those of colloids particles, their dispersions are called macromolecular colloids, e.g., proteins, starch, and cellulose structure macromolecular colloids.
Related colloids (Micelles): Those colloids which carry on as ordinary, solid electrolytes at low fixations, however, show colloidal properties at higher concentrations because of the arrangement of amassed particles of colloidal dimensions. Such substances are likewise alluded to as related colloids.
Kraft Temperature (Tk): Micelles are framed uniquely over a specific temperature called Kraft temperature.
Basic Micelle Concentration (CMC): Micelles are shaped uniquely over a specific focus called basic micelle fixation.
Cleansers: These are sodium or potassium salts of higher unsaturated fats, e.g., sodium stearate CH3(CH2)16COO-Na+
(Surface Chemistry: Class 12 )
Techniques for the planning of colloids:
Synthetic techniques: Colloids can be set up by substance reactions leading to the development of molecules. These molecules total, leading to an arrangement of sols.
Electrical disintegration or Bredig’s Arc technique: In this strategy, the electric bend is struck between the metal submerged electrodes in the dispersion medium. The extraordinary warmth delivered disintegrates the metal, which at that point, gathers to shape particles of colloidal size.
Peptization: It is the way toward changing over an accelerated into colloidal sol by shaking it with dispersion medium within sight of a limited electrolyte quantity. The electrolyte utilized for this reason for existing is called the peptizing operator.
Purification of colloids:
Dialysis: It is a cycle of eliminating a dissolved substance from a colloidal solution by methods for diffusion through a reasonable layer.
Electrodialysis. The cycle of dialysis is very moderate. It very well may be made quicker by applying an electric field if the dissolved substance in the debased colloidal solution is just an electrolyte.
Ultrafiltration: It is the way toward isolating the colloidal particles from the dissolvable and solvent solutes present in the colloidal solution by extraordinarily arranged channels, which are penetrable to all substances aside from the colloidal particles.
Ultracentrifugation: In this cycle, the colloidal solution is taken in a cylinder set in an ultracentrifuge. On turning the cylinder at extremely rapid, the colloidal particles settle down at the cylinder base, and the pollutions stay in solution. The settled particles are blended in with the dispersion medium to recover the sol.
Properties of colloids: Positively charged colloidal particles:(i) These incorporate hydrated metallic oxides, such as Fe2O3.H2O, Cr2O3.H2O, Al2O3.H2O.
(ii) Basic color stuff like malachite green, methylene blue sols.
(iii) Example – Hemoglobin (blood).Negatively charged colloidal particles:(i) Metallic sulfides like As2S3, Sb2S3 sols.
(ii) Acid color stuff like eosin, methyl orange, Congo red sols.
(iii) Examples – Starch sol, gum, gelatin, earth, charcoal, egg whites, etc.
Shading: The shade of colloidal solution relies on the frequency of light dispersed by the colloidal particles, which, like this, relies on the nature and size of particles. The shading additionally relies on the way wherein light is gotten by the eyewitness.
Brownian movement: Colloidal particles move in zig – zoom way. This sort of movement is expected to collide molecules of dispersion medium continually with colloidal particles.
Colligative properties: The estimations of colligative properties (osmotic pressure, lowering in fume pressure, gloom in freezing point, and rise in boiling point) are of little order when contrasted with values appeared by true solutions at similar focuses.
Tyndall impact: The scattering of light emission by colloidal particles is called Tyndall impact. The splendid cone of light is known as the Tyndall cone.
Charge on colloidal particles: Colloidal particles consistently convey an electric charge. This charge’s idea is the equivalent of all the particles in a given colloidal solution and might be either certain or negative.
Helmholtz electrical twofold layer: When the colloidal particles obtain negative or positive charge by selective adsorption of one of the particles, it draws in counter particles from the medium framing a subsequent layer. The mix of these two layers of inverse charges around colloidal particles is called Helmholtz electrical twofold layer.
Electrokinetic potential or zeta potential: The potential difference between the fixed and diffused layers of inverse charges is called electrokinetic potential or zeta potential.
Electrophoresis: The movement of colloidal particles under an applied electric potential is called electrophoresis.
Coagulation or precipitation: The way toward settling colloidal particles as encouraged is called coagulation.
Hardy – Schulze rules:
- I) Oppositely charged particles are successful for coagulation.
- ii) The coagulating intensity of electrolyte increments with increment in control of the particles utilized for coagulation. Models – Al3+> Ba2+> Na+ for adversely charged colloids. Fe (CN)6]4->>>Cl– for positively charged colloids.
Sorts of emulsions:
Water dispersed in oil: When water is the dispersed phase and oil is the dispersion medium. For example spread
Oil dispersed in water: On the off chance, when the oil is the dispersed phase and water is the dispersion medium. For example milk
Emulsification: It is the way toward settling an emulsion by methods for an emulsifier.
Emulsifying specialist or emulsifier: These are the substances that are added to balance out the emulsions. Models – cleansers, gum
Demulsification: It is the way toward breaking an emulsion into its constituent liquids freezing, boiling, centrifugation, or concoction techniques.
Questions:
Q.State whether the accompanying proclamations are True or False.
- The adsorption isotherm is a bend that communicates the variety in the measure of gas adsorbed by the adsorbent with the temperature at a steady pressure.
- Freundlich isotherm fizzles at high pressure.
- On the off chance that the plot of log x/m on the y-hub and log P on the x-pivot is a straight line, at that point, Freundlich isotherm is substantial.
- Freundlich isotherm clarifies the conduct of adsorption precisely.
- In the condition – x/m = k.P1/(n > 1), ‘k’ and ‘n’ are constants that rely upon the idea of the adsorbent and the gas at a specific temperature.
Solutions:
- Bogus
- True
- True
- Bogus
- True
(Surface Chemistry: Class 12)

