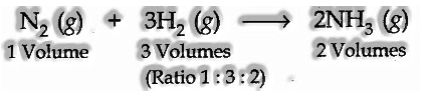Some Basic Concepts of Chemistry: Class 11 Chemistry NCERT Chapter 1
Key Features of NCERT Material for Class 11 Chemistry Chapter 1 – Some Basic Concepts of Chemistry
In this chapter: Some basic concepts of chemistry, you will learn about applications , laws and concepts of chemistry. You reasonably have an unpleasant thought of what atoms and molecules are. In any case, did you ever ponder that atomic mass and molecular mass are significant ideas too? What do we do by knowing the mass of such small atoms and molecules? Indeed, the hugeness of these atomic and molecular masses is gigantic! In this section, we will cover the ideas and furthermore perceive how significant they are.
An atom is such little a molecule that it can’t be seen or secluded. In this way, it is difficult to decide the genuine mass of a solitary atom by gauging it. In any case, Avogadro’s theory, at last, tackled the issue. He took equivalent volumes of two unique gases under comparable states of temperature and pressure. He at that point gauged them.
Shockingly, the ratio of their masses was equivalent to the ratio of their single molecules. Along these lines, however, the genuine masses of the atoms couldn’t be resolved, their relative masses could be resolved. On the off chance that the atomic mass of hydrogen is taken as 1, the relative atomic mass of oxygen is 16.
At first, scientists acquired the atomic masses of the considerable number of elements by contrasting and the mass of hydrogen taken as 1. In any case, the issue was that the atomic masses of a large portion of the elements came out to be fractional in this technique. In this way, carbon is taken as the reference for the assurance of atomic masses.
Quick revision notes
Importance of Chemistry
Science directly affects our life and has a wide scope of utilization in various fields. These are given beneath:
(A) In Agriculture and Food:
(I) It has given chemical fertilizers, for example, urea, calcium phosphate, sodium nitrate, ammonium phosphate and so forth.
(ii) It has assisted with shielding the harvests from insects and unsafe microscopic organisms, by the utilization of certain successful bug sprays, fungicides and pesticides.
(iii) The utilization of preservatives has assisted with saving food items like jam, spread, squashes and so forth for longer periods.
(B) In Health and Sanitation:
(I) It has furnished humankind with an enormous number of life-sparing drugs. Today, dysentery and pneumonia can be cured because of the revelation of sulpha drugs and penicillin life-sparing drugs. Cisplatin and taxol have been seen as extremely successful for malignant growth treatment and AZT (Azidothymidine) is utilized for AIDS casualties.
(ii) Disinfectants, for example, phenol are utilized to end the microorganisms present in channels, latrine, floors and so on.
(iii) A low concentration of chlorine i.e., 0.2 to 0.4 parts per million (ppm) is utilized ‘ for cleansing of water to make it suitable for drinking purposes.
(C) Saving the Environment:
The quick industrialisation everywhere throughout the world has brought about part of contamination.
Toxic gases and chemicals are by and large continually delivered in the air. They are contaminating the environment at a disturbing rate. Scientists are working day and night to create substitutes which may cause lower pollution. For instance, CNG (Compressed Natural Gas), a substitute for petrol, is compelling in checking contamination brought about via vehicles.
(D) Application in Industry:
Science has assumed a significant job in creating numerous mechanically ^ produced fertilizers, antacids, acids, salts, colours, polymers, drugs, soaps,
Soaps, metal compounds and other inorganic and organic chemicals incorporating new materials contribute in a major manner to the national economy.
Matter
Anything which has mass and consumes space is called matter.
For instance, book, pencil, water, air are made out of matter as we realize that they have mass and they consume space.
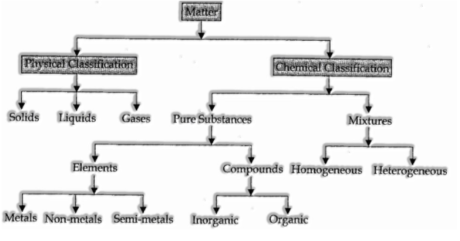
Classification of Matter
There are two different ways of characterizing the matter:
(A) Physical characterization
(B) Chemical characterization
(A) Physical Classification:
The matter has 3 physical states:
- Solids 2. Liquids 3. Gases
Solids:
The particles are held extremely near one another in a systematic manner and there isn’t a lot of opportunity of development.
Qualities of solids: Solids have positive volume and unequivocal shape.
Liquids:
In liquids, the particles are near one another yet can move around. Qualities of liquids: Liquids have unmistakable volume yet not clear shape.
Gases:
In gases, the particles are far separated when contrasted with those present in strong or fluid states. Their development is simple and quick.
Attributes of Gases: Gases have neither clear volume nor unequivocal shape. They totally involve the holder wherein they are put.
(B) Chemical Classification:
In view of the organization, the matter can be isolated into two fundamental sorts:
- Pure Substances 2. Mixtures.
- Pure substances: A pure substance might be characterized as a solitary substance (or matter) which can’t be isolated by basic physical techniques.
Pure substances can be additionally delegated (I) Elements (ii) Compounds
(I) Elements:
An element comprises just one kind of particle. Those particles could be atoms or molecules.
For instance, sodium, copper, silver, hydrogen, oxygen and so forth are a few instances of elements. They all contain atoms of one sort. Be that as it may, atoms of various elements are distinctive in nature. A few elements, for example, sodium . or then again copper contains single atoms held together as their constituent particles while in some others at least two atoms join to give molecules of the element. In this way, hydrogen, nitrogen and oxygen gases comprise molecules in which two atoms join to give the separate molecules of the element.
(ii) Compounds:
It might be characterized as a pure substance containing at least two elements consolidated together to a fixed extent by weight and can be disintegrated into these elements by appropriate chemical techniques. In addition, the properties of a compound are out and out unique in relation to the comprising elements.
The compounds have been characterized into two sorts. These are:
(I) Inorganic Compounds: These are compounds which are acquired from non-living sources, for example, rocks and minerals. A couple of models are Common salt, marble, gypsum, washing soft drink and so forth.
(ii) Organic Compounds are the compounds which are available in plants and creatures. All the organic compounds have been found to contain carbon as their basic constituent. For instance, sugars, proteins, oils, fats and so on.
Mixtures:
The mix of at least two elements or compounds which are not chemically consolidated together and may likewise be available to any extent, is called a mixture. A couple of instances of mixtures are milk, ocean water, petrol, lime water, paint glass, concrete, wood and so forth.
Sorts of mixtures: Mixtures are of two kinds:
(I) Homogeneous mixtures:
A blend is supposed to be homogeneous in the event that it has a uniform piece all through and there are no obvious limits of separation between the constituents.
For instance: A blend of sugar arrangement in water has a similar sugar water part all through and all parts have similar pleasantness.
(ii) Heterogeneous mixtures:
A mixture is supposed to be heterogeneous in the event that it doesn’t have a uniform piece all through and has obvious limits of separation between the different constituents. The various constituents of a heterogeneous blend can be seen even with naked eyes.
For instance: When iron filings and sulfur powder are combined, the blend shaped is heterogeneous. It has the greyish-yellow appearance and the two constituents, iron and sulfur, can be handily related to unaided eyes.
Differences among Compounds and Mixtures
Compounds
- In a compound, at least two elements are joined chemically.
- In an aggravating way, the elements are available in the fixed ratio by mass. This ratio can’t change.
- Compounds are consistently homogeneous i.e., they have the same synthesis all through.
- In a compound, constituents can’t be isolated by physical techniques
- In an intensifier, the constituents lose their personalities i.e., I compound doesn’t show the attributes of the comprising elements.
Mixtures
- In a blend, or more elements or compounds are essentially blended and not consolidated chemically.
- In a blend, the constituents are absent in a fixed ratio. It can differ
- Mixtures might be either homogeneous or heterogeneous in nature.
- Constituents of mixtures can be isolated by physical techniques.
- In a blend, the constituents don’t lose their personalities i.e., a blend shows the attributes of the considerable number of constituents.
We have talked about the physical and chemical order of matter. A portrayal of the equivalent is given beneath.
Properties of Matter and Their Measurements
Physical Properties:
Those properties which can be estimated or seen without changing the personality or the arrangement of the substance.
A few instances of physical properties are shading, scent, liquefying point, melting point and so forth. Chemical Properties: It requires a chemical change to happen. The instances of chemical properties are trademark responses of various substances. These incorporate acidity, basicity, instability and so on.
Units of Measurement
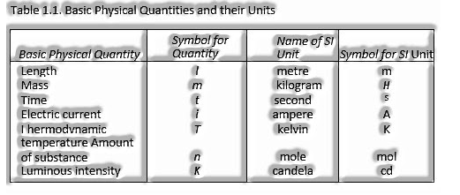
Basic Units: The amounts mass, length and time are called crucial amounts and their units are known as basic units.
There are seven fundamental units of measurement for the amounts: length, mass, time, temperature, a measure of substance, electric flow and luminosity.
Si-System: This arrangement of measurement is the most widely recognized framework utilized all throughout the world.
It has given units of all the seven fundamental amounts recorded previously.
Definitions of Basic SI Units
- Meter: It is the length of the path that went by light in vacuum during a period timespan/299792458 of a second.
- Kilogram: It is the unit of mass. It is equivalent to the mass of the universal model of the kilogram. ,
- Second: It is the duration of 9192631, 770 times of radiation which relate to the progress between the two hyperfine degrees of the ground condition of the caesium-133 atom.
- Kelvin: It is the unit of thermodynamic temperature and is equivalent to 1/273.16 of the thermodynamic temperature of the triple purpose of water.
- Ampere: The ampere is that steady current which whenever kept up in two straight equal conductors of unbounded length, of the irrelevant roundabout cross area and put, 1 meter separated in a vacuum, would deliver between these conductors a power equivalent to 2 x 10-7 N for every meter of length.
- Candela: It might be characterized as the luminous force in a provided guidance, from a source which transmits monochromatic radiation of frequency 540 x 1012 Hz and that has a radiant power via that path of 1/683 watt for each steradian.
- Mole: It is the measurement of substance which contains the same number of elementary elements as there are atoms in 0.012 kilograms of carbon – 12. Its symbol is ‘mol’.
Mass and Weight
Mass:
Mass of a substance is the measure of matter present in it.
The mass of a substance is consistent.
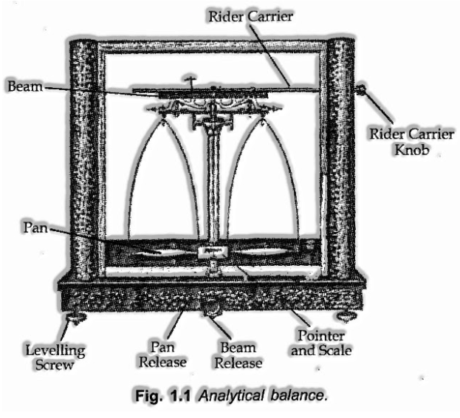
The mass of a substance can be resolved precisely in the research centre by utilizing a scientific balance.
SI unit of mass = kilogram.
 Weight:
Weight:
It is the power applied by gravity on an item. Weight of substance may shift starting with one spot then onto the next because of progress in gravity.
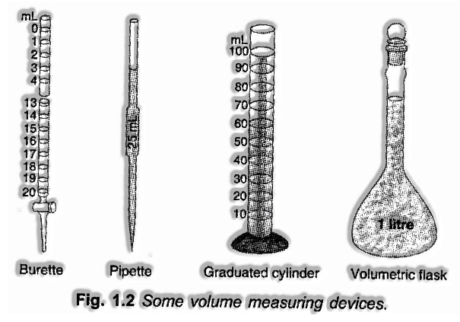
Volume: Volume implies the space involved by matter. It has units (cubic of length). In SI units, the volume is communicated in metre3 (m3). Be that as it may, a well-known unit of estimating volume, especially in liquids is the liter (L) however it isn’t in SI units or an S.I. unit.
Scientifically,
1L is 1000 mL is 1000 cm3 is 1dm3.
The volume of liquids can be estimated by various gadgets like burette, pipette, chamber, estimating jar and so on. Every one of them has been aligned.
Temperature:
There are three scales in which temperature can be estimated. These are known as the Celsius scale (°C), Fahrenheit scale (°F) and Kelvin scale (K).
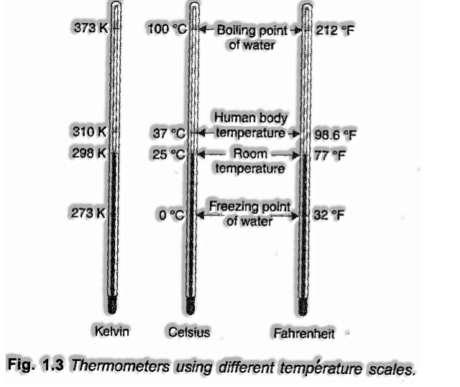
– > Thermometres with Celsius scale is aligned from 0°C to 100°C.
– > Thermometres with Fahrenheit scale is adjusted from 32°F to 212°F.
– > Kelvin’scale of temperature is S.I. scale and is regular these days.The temperature on this scale is by the indication K.
The temperature on two scales are identified with one another by the relationship
Density:
Density of a substance is its measure of mass per unit volume. Along these lines, the SI unit of density can be gotten as follows:

This unit is very huge and a physicist frequently communicates density in g cm3 where mass is communicated in gram and volume is communicated in cm3.
Uncertainty in Measurements
Every scientific measurement includes a certain level of mistake or vulnerability. The blunders which emerge rely on two variables.
(I) Skill and exactness of the one who measures (ii) Limitations of estimating instruments.
Scientific Notation
It is exponential documentation wherein any number can be symbolized in the structure N x 10n where n is a type having positive or negative qualities and N can change between 1 to 10. Accordingly, 232.508 can be composed as 2.32508 x 102 in scientific documentation.
Presently let us perceive how calculations are completed with numbers communicated in scientific documentation.
Calculation including multiplication and division
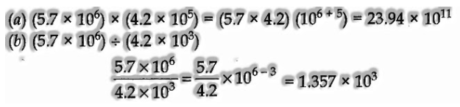
Calculation including addition and subtraction:
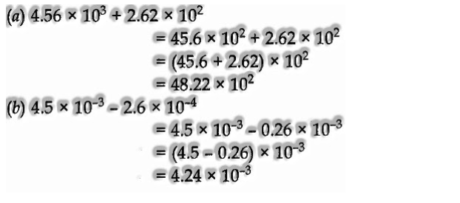
For these two operations, the principal numbers are written so that they have a similar type. From that point onward, the coefficients are included or deducted by and large. For instance,
-
Significant Figures
Huge figures are important digits which are known with conviction. There are sure principles for deciding the number of critical figures. These are expressed underneath:
- All non-zero digits are noteworthy. For instance, in 285 cm, there are three noteworthy figures and in 0.25 mL, there are two figures.
- Zeros going before the first non-zero digit are not noteworthy. Such zeros demonstrate the situation of decimal points. For instance, 0.03 has one figure and 0.0052 has two figures.
- Zeros between two non-zero digits are important. Along these lines, 2.005 has four critical figures.
- Zeros toward the end or right of a number are significant given they are on the correct side of the decimal point. For instance, 0.200 g has three critical figures.
- Checking quantities of items. For instance, 2 balls or 20 eggs have interminable noteworthy figures as these are definite numbers and can be written to by composing an unbounded number of zeros in the wake of putting a decimal. i.e., 2 = 2.000000 or on the other hand 20 = 20.000000
-
Addition and Subtraction of Significant Figures
In addition or subtraction of the numbers having various precisions, the conclusive outcome ought to be accounted for to an indistinguishable number of decimal spots from in the term having a minimal number of decimal spots.
For instance, let us complete the addition of three numbers 3.52, 2.3 and 6.24, having various precisions or distinctive numbers of decimal spots.
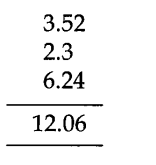
The conclusive outcome has two decimal places yet the appropriate response must be accounted for just up to one decimal spot, i.e., the appropriate response would be 12.0.
Subtraction of numbers should be possible similarly as the addition.

The conclusive outcome has four decimal spots. Be that as it may, it must be accounted for just up to two decimal spots, i.e., the appropriate response would be 11.36.
-
Multiplication and Division of Significant Figures
In the multiplication or division, the conclusive outcome ought to be accounted for up to an indistinguishable number of critical figures from present at all exact numbers.
Multiplication of Numbers ie 2.2120 x 0.011 = 0.024332
As per the standard, the conclusive outcome = 0.024
Division of Numbers: 4.2211÷3.76 = 1.12263
The right answer = 1.12
- Dimensional Analysis
Frequently while ascertaining, there is a need to change over units from one framework to another. The technique used to achieve this is called factor label strategy or unit factor strategy or dimensional examination.
Laws of Chemical Combinations
The mix of elements to frame compounds is represented by the accompanying five essential laws.
(I) Law of Conservation of Mass
(ii) Law of Definite Proportions
(iii) Law of Multiple Proportions
(iv) Law of Gaseous Volume (Gay Lussac’s Law)
(v) Avogadro’s Law
(I) Law of Conservation of Mass
The law was built up by a French scientific expert, A. Lavoisier. The law states:
In all physical and chemical changes, the all-out mass of the reactants is equivalent to that of the items.
As it were, the matter can nor be made nor pulverized.
The accompanying tests represent the reality of this law.
(a) When matter experiences a physical change.
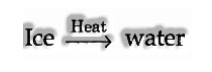
It is discovered that there is no adjustment in weight however a physical change has occurred.
(b) When matter experiences a chemical change.
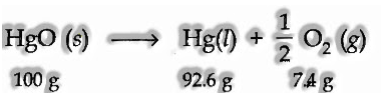
For instance, deterioration of mercuric oxide.
During the above disintegration response, the matter is neither increased nor lost.
(ii) Law of Definite Proportions
As indicated by this law:
A pure chemical compound consistently comprises similar elements joined together to a fixed extent by weight.
For instance, Carbon dioxide might be shaped in various manners i.e.,
(iii) Law of Multiple Proportions
In the event that two elements consolidate to shape at least two compounds, the weight of one of the elements which join with a fixed weight of the other in these compounds bears straightforward entire number ratio by weight.
For instance,
(iv) Gay Lussac’s Law of Gaseous Volumes
The law expresses that, under comparable states of temperature and pressure, at whatever point gases join, they do as such in volumes which bear a basic entire number ratio with one another and furthermore with the vaporous items. The law might be outlined by the accompanying models.
(a) The combination among hydrogen and chlorine:
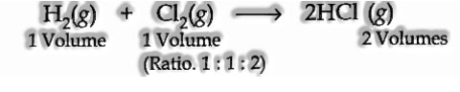
(b) Combination among nitrogen and hydrogen: The two gases lead to the development of smelling salts gas under appropriate conditions. The chemical condition is
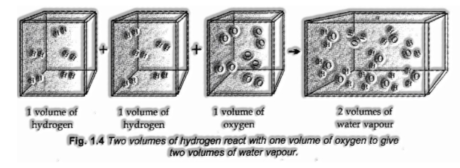
(v) Avogadro’s Law:
Avogadro recommended that equivalent volumes of gases at a similar temperature and pressure ought to contain an equivalent number of molecules.
For instance,
In the event that we think about the response of hydrogen and oxygen to deliver water, we see that two volumes of hydrogen consolidate with one volume of oxygen to give 2 volumes of water without leaving any unreacted oxygen.
Dalton’s Atomic Theory
In 1808, Dalton distributed ‘A New System of Chemical Philosophy’ where he proposed the accompanying:
- Matter comprises inseparable atoms.
- All the atoms of a given element have indistinguishable properties including indistinguishable mass. Atoms of various elements contrast in mass.
- Compounds are shaped when atoms of various elements consolidate in a fixed ratio.
- Chemical responses include revamping of atoms. These are neither made nor demolished in a chemical response.
Atomic Mass
The atomic mass of an element is the occasion an atom of that element is heavier than an atom of carbon taken as 12. It might be noticed that the atomic masses as stated above are the relative atomic masses and not the real masses of the atoms.
One atomic mass unit (AMU) is equivalent to l/twelfth of the mass of an atom of the carbon-12 isotope. It is otherwise called a unified mass.
Normal Atomic Mass
The majority of the elements exist as isotopes which are various atoms of a similar element with various mass numbers and the equivalent atomic number. Subsequently, the atomic mass of an element must be its normal atomic mass and it might be characterized as the normal relative mass of an atom of an element when contrasted with the mass of carbon atoms (C-12) taken as 12w.
Molecular Mass
Molecular mass is the total sum of atomic masses of the elements in a molecule. It is gotten by increasing the atomic mass of every element by a number of its atoms and including them together.
For instance,
The molecular mass of methane (CH4)
= 12.011 u + 4 (1.008 u)
= 16.043 u
Formula Mass
Ionic compounds, for example, NaCl, KNO3, Na2C03 and so forth don’t comprise of molecules i.e., single substances yet exist “as particles firmly stuffed together in a three-dimensional space as appeared in – Fig. 1.5.
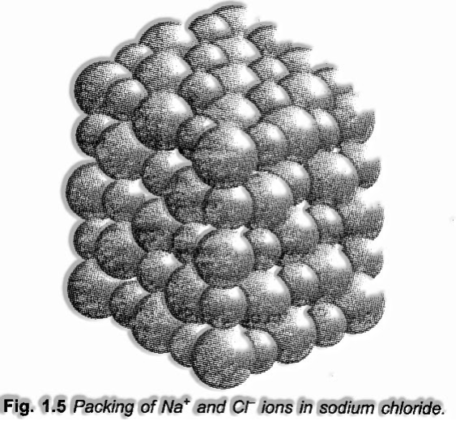
In such cases, the formula is utilized to ascertain the formula mass rather than molecular mass. In this manner, the formula mass of NaCl = Atomic mass of sodium + atomic mass of chlorine
Is equal to 23.0 u + 35.5 u = 58.5 u.
-
Mole Concept
It is discovered that one gram atom of any element contains a similar number of atoms and one gram molecule of any substance contains a similar number of molecules. This number has been tentatively decided and seen as equivalent to 6.022137 x 1023 The worth is for the most part called Avogadro’s number or Avogadro’s steady.
It is typically spoken to by NA:
Avogadro’s Number, NA = 6.022 × 1023
-
Percentage Composition

One can check the immaculateness of a given example by breaking down this information. Let us comprehend by taking the case of water (H20). Since water contains hydrogen and oxygen, the rate creation of both these elements can be determined as follows:
-
Empirical Formula
The formula of the compound which gives the simplest entire number ratio of the atoms of various elements present in one molecule of the compound.
For instance, the formula of hydrogen peroxide is H202. So as to communicate its empirical formula, we need to take out a typical factor 2. The simplest entire number ratio of the atoms is 1:1 and the empirical formula is HO. Likewise, the formula for glucose is C6H1206. So as to get the simplest entire number of the atoms,
Basic factor = 6
So, the ratio is = 1 : 2 : 1 The empirical formula of glucose = CH20
-
Molecular Formula
The formula of a compound which presents the genuine ratio of the atoms of different elements present in one molecule of the compound.
For instance, molecular formula of hydrogen peroxide = H202and Glucose = C6H1206
Molecular formula = n x Empirical formula
Where n is the basic factor and furthermore called an increasing variable. The estimation of n might be 1, 2, 3, 4, 5, 6 and so forth.
In the event that n is 1, Molecular formula of a compound = Empirical formula of the compound.
-
Stoichiometry and Stoichiometric Calculations
The word ‘stoichiometry’ is gotten from two Greek words—Stoicheion (which means element) and metron (which means measure). Stoichiometry, in this way, manages the calculation of masses (once in a while volume additionally) of the reactants and the items associated with a chemical response. Let us think about the ignition of methane. A reasonable condition for this response is as given beneath:
Restricting Reactant/Reagent
Some of the time, in alchemical condition, the reactants present are not the sum as required by the reasonable condition. The measure of items shaped at that point relies on the reactant which has responded totally. This reactant which response totally in the response is known as the restricting reactant or constraining reagent. The reactant which isn’t expended totally in the response is called abundance reactant.
Responses in Solutions
At the point when the responses are done in arrangements, the measure of substance present in its given volume can be communicated in any of the accompanying ways:
- Weight per cent (w/w%) or Mass per cent
- Mole fraction
- Molarity
- Molality
Mass per cent: It is acquired by utilizing the accompanying connection:
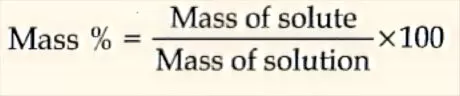
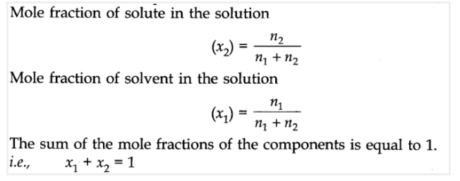
Mole fraction: It is the ratio of a number of moles of a specific segment to the all outnumbers of moles of the arrangement. For an answer containing n2 moles of the solute broke up in n1 moles of the dissolvable,
Molarity: It is characterized as the quantity of moles of solute in 1 litre of the arrangement.

Molality: It is characterized as the quantity of moles of solute present in 1 kg of dissolvable. It is meant by m.
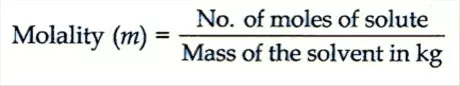
- All substances contain matter which can exist in three states — strong, fluid or gas.
- Matter can likewise be grouped into elements, compounds and mixtures.
- Element: An element contains particles of just one sort which might be atoms or molecules.
- Compounds are formed when atoms of at least two elements join in a fixed ratio to one another.
- Mixtures: Many of the substances existing in our surrounding are mixtures.
- Scientific documentation: The measurement of amounts in science are spread over a wide range of 10-31to 1023. Subsequently, a helpful arrangement of communicating the number in scientific documentation is utilized.
- Scientific figures: The vulnerability is dealt with by determining the quantity of critical figures wherein the perceptions are accounted for.
- Dimensional examination: It assists in communicating the deliberate amounts in various frameworks of units.
Laws of Chemical Combinations are:
(I) Law of Conservation of Mass
(ii) Law of Definite Proportions
(iii) Law of Multiple Proportions
(iv) Gay Lussac’s Law of Gaseous Volumes
(v) Avogadro’s Law.
- Atomic mass: The atomic mass of an element is communicated relative to 12C isotope of carbon which has a precise estimation of 12u.
- Average atomic mass: Obtained by considering the regular abundance of various isotopes of that element.
- Molecular mass: The molecular mass of a molecule is acquired by taking the aggregate of atomic masses of various atoms present in a molecule.
- Avogadro number: The quantity of atoms, molecules or some other particles present in a given framework is communicated as far as Avogadro constant. = 6.022 x 1023
- Balanced chemical condition: A decent condition has a similar number of atoms of every element on the two sides of the condition.
- Stoichiometry: The quantitative investigation of the reactants required or the items framed is called stoichiometry. Utilizing stoichiometric calculations, the measures of at least one reactant required to deliver a specific measure of the item can be resolved and the other way around.
Questions
Q: The particular heat of metal of atomic mass 32 is probably going to be:
0.25
0.24
0.20
0.15
Answer: Specific heat= 6.4/atomic mass
In this manner, Specific Heat = 6.4/32 = 0.2
Consequently, the appropriate response is (c).
In the next chapter of NCERT class 11 chemistry: Structure of Atom you will learn about parts of atoms and different theories associated with the discovery of the atom.


 Weight:
Weight: