Redox Reactions: Class 11 Chemistry NCERT Chapter 8

Key Features of NCERT Material for Class 11 Chemistry Chapter 8 – Redox Reaction
In the last chapter 7, you learned about chemical equilibrium. In this chapter: Redox Reaction, you will learn about that do you understand that a redox reaction may be going on around you at the present time? Truly! The rusting of iron, consuming, and rotting a substance are only redox reactions. In any case, what really occurs in a redox reaction? What does the term ‘redox’ even mean? Would you like to know? At that point, jump on this train of redox reactions and begin investigating the articles underneath.
Redox Reactions
The decrease is the increase of electrons, while oxidation is the loss of electrons. The mix of decrease and oxidation reaction together alludes to the redox reaction/redox process. As talked about, it is essential to comprehend “adjusting redox reactions.”
There are commonly two techniques for adjusting redox reactions (chemical conditions) in a redox procedure: the two techniques are-Oxidation Number Method and Half-Reaction Method.
Quick update notes :
-
Oxidation
Oxidation is characterized as the expansion of oxygen/electronegative element to a substance or removal of hydrogen/electropositive element from a substance.
For instance

-
Reduction
The decrease is characterized as the removal of oxygen/electronegative element from a substance or expansion of hydrogen or electropositive element to a substance.
For instance
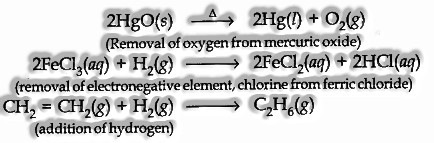
- Redox Reaction in Terms of Electron Transfer Reaction
A couple of instances of redox reaction based on the electronic idea are given beneath:
As per electronic idea, each redox reaction comprises two stages known as half-reactions.
(I) Oxidation reaction: Half reactions that include loss of electrons are called oxidation reactions.
(ii) Reduction reaction: Half reactions that include the addition of electrons are called decrease reactions.
Oxidising agent: Acceptor of electrons.
Reducing agent: Donar of electrons.
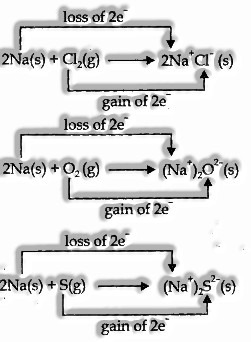
-
Competitive Electron Transfer Reactions
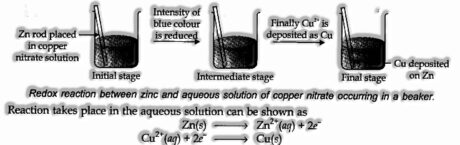
To comprehend this idea, let us do an investigation.
Spot a segment of metallic zinc in a fluid arrangement of copper nitrate, as appeared in Fig. Following one hour following changes will be taken note.
(I) Strips get covered with rosy metallic copper.
(ii) The blue shade of the arrangement vanishes.
(iii) If hydrogen sulfide gas is gone through the arrangement, the appearance of white ZnS can be – seen on making the arrangement alkaline with smelling salts.
-
Oxidation Number
It is the oxidation condition of an element in a compound which is the charge allocated to an iota of a compound is equivalent to the number of electrons in the valence shell of a molecule that is picked up or lost totally or to a considerable degree by that particle while framing a bond in a compound.
-
Rules for Assigning Oxidation Numbers
(I) The element’s oxidation number in its elementary structure is zero.
For instance, H2, 02, N2, and so forth have oxidation numbers equivalent to zero.
(ii) In a solitary monatomic particle, the oxidation number is equivalent to the charge on the particle. For instance, Na+ particles have an oxidation number of +1, and Mg2+ particles have +2.
(iii) Oxygen has an oxidation number – 2 in its compounds. In any case, there are a few special cases.
Compounds, for example, peroxides. Na202, H202
the oxidation number of oxygen = – 1 In OF2
O.N. of oxygen = +2 02F2
O.N. of oxygen = +1
(iv) In non-metallic compounds of hydrogen-like HCl, H2S, H2O oxidation number of hydrogen = + 1 however in metal hydrides oxidation number of hydrogen = – 1
[LiH, NaH, CaH2, etc.]
(v) In compounds of metals and non-metals have a positive oxidation number while non-metals have a negative oxidation number, for instance, In NaCl. Na has a +1 oxidation number, while chlorine has – 1.
(vi) If there are two non-metallic iotas in a compound, the molecules with high electronegativity are doled out negative oxidation numbers, while different particles have positive oxidation numbers.
(vii) The logarithmic whole of the oxidation number of all iotas in a compound is equivalent to zero.
(viii) In a polynuclear particle, the whole of the oxidation no. of the considerable number of molecules in the particle is equivalent to the net charge on the particle.
For instance, in (C03)2—Sum of carbon molecules and three oxygen iotas is equivalent to – 2.
Fluorine (F2) is so profoundly responsive non-metal that it uproots oxygen from water.
![]()
Disproportionation Reaction. In a disproportionation reaction, an element in one oxidation state is simultaneously oxidized and reduced.
![]()
Henceforth, the oxygen of peroxide, which is available in – 1 oxidation state, is associated with zero oxidation state and in 02 and in H2O diminishes to – 2 oxidation state.
-
Fractional Oxidation Numbers
Elements, as such, don’t have any partial oxidation numbers. When a similar element is engaged with various bonding in an animal category, their genuine oxidation states are entire numbers; however, a normal of these are partial.
For instance, In C302.
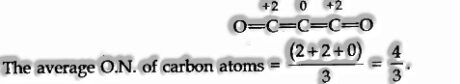
Partial O.N. of a specific element can be calculated just on the off chance that we think about the structure of the compound or in which it is available.
- Balancing of Redox Reactions
(I) Oxidation Number Method. Following advances are included:
(ii) Write the right recipe for every reactant and item.
(b) By doling out the oxidation change in oxidation number can be distinguished.
(c) Calculate the expansion and decline in the oxidation number per particle as for the reactants, on the off chance that more than one particle is available, at that point, duplicate by a suitable coefficient.
(d) Balance the condition regarding all iotas. Equalization hydrogen and oxygen molecules moreover.
(e) If the reaction is completed in an acidic medium, use H+ particles in the condition. On the off chance that it is in fundamental medium use OF–particles.
(f) Hydrogen particles in the articulation can be adjusted by including (H20) atoms to the reactants or items.
In the event that there is a similar number of oxygen molecules on both sides of the condition, it speaks to the fair redox reaction.
(ii) Half Reaction Method. In this strategy, two half conditions are adjusted independently and then included to give adjusted condition.
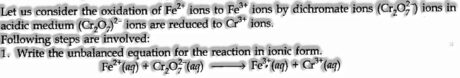
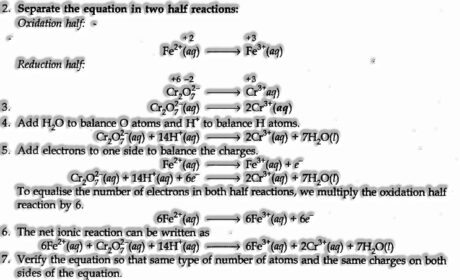
-
Redox Reactions as the Basis for Titration
Potassium Permanganate Titration: In these titrations, potassium permanganate (pink in shading) goes about as an oxidizing operator in the acidic medium. In contrast, oxalic corrosive or some ferrous salts goes nearly as a reducing specialist.
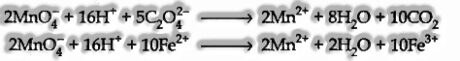
These are the instances of redox titration.
On both these titrations, potassium permanganate itself goes about as a marker. It is usually known as self pointer. The presence of pink shading in the arrangement speaks to the end focus.
Potassium Dichromate Titration: instead of potassium permanganate, potassium dichromate can likewise be utilized within the sight of dil. H2S04. The ionic condition for the redox reaction with FeS04 (Fe2+ particles) is given.

-
Limitation of Concept of Oxidation Number
As indicated by the idea of oxidation number, oxidation implies increment in oxidation number – by loss of electrons and decrease means decline in oxidation number by the addition of electrons. Be that as it may, during oxidation, there is a decline in electron density, while increment in electron density around the molecule experiencing decreases.
-
Redox Reactions and Electrode Processes—Electrochemical Cells
A gadget wherein the redox reaction is conveyed in a roundabout way, and the abatement in vitality shows up as the electrical vitality is called electrochemical cells.
Electrolytic Cell. The cell where electrical vitality is changed over into chemical vitality. Model, when the lead stockpiling battery is revived, goes about as electrolytic.
Redox Reactions and Electrode Processes. At the point when the zinc pole is plunged in copper sulfate arrangement, redox reaction starts; thus, zinc is oxidized to Zn2+ particles, and Cu2+ particles are decreased to metal.
- Redox reaction. Reactions in which oxidation and decrease happen at the same time are called redox reactions.
- Oxidation. Includes the loss of at least one electron.
- Reduction. Includes the addition of at least one electron.
- Oxidizing specialist. Tolerating electrons.
- Reducing specialist. Losing electrons.
- Electrochemical cell. It is a gadget where redox reaction is conveyed by implication, and reduction in vitality gives electrical vitality.
- Electrode potential. It is the possible contrast between the anode and its particles in an arrangement.
- Standard cathode potential. It is the capability of a terminal as for standard hydrogen cathode.
- Electrochemical arrangement. It is a movement arrangement. It has been framed by orchestrating the metals arranged by expanding standard decrease possible worth.
Questions
Q: Determine the species experiencing the oxidation and decrease
Arrangement: In the above reaction, H2S is oxidized in light of the more electronegative element’s nearness in the response. Along these lines, the expansion of chlorine to hydrogen happens, and a more electropositive element “hydrogen” expulsion occurs from sulfur. In this way, chlorine is diminished because of the expansion of hydrogen.

