Periodic Classification of Elements:Class 10 Science NCERT Chapter 5

Key Features of NCERT Material for Class 10 Science Chapter 5 – Periodic Classification of Elements
In the previous chapter 4:Carbon and its compound,we have learned about the properties of carbon compound and the basics of organic chemistry.In this chapter 5: Periodic Classification of Elements we will sudy about the arrangement of elements in the periodic table.
Quick revision notes
“The periodic table is a plain technique for showing the components in such a manner, that the components having comparable properties happen in a similar vertical segment or gathering”.
Prior endeavors of the grouping of components: Dobereiner’s Triads, Newland’s law of octaves.
Dobereiner’s Triads: This grouping depends on the atomic mass. As per this, when components are organized arranged by expanding atomic masses, gatherings of three components, having comparative properties are acquired. The atomic mass of center component of the ternion being almost equivalent to the normal of the atomic masses of the other two components.
For Example Li (6.9), Na (23), K (39).
Impediment: It neglects to organize all the known components as triads, in any event, having comparative properties.
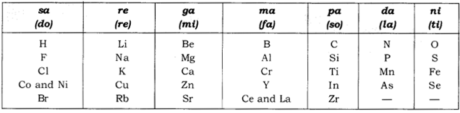
Newland’s Law of Octaves: According to this ‘when components are submitted in request of expanding atomic masses, the physical and substance properties of each eighth component are a redundancy of the properties of the primary component.’
Type of Newland’s octaves is given in the accompanying table:
Limitations
- Law of octaves was pertinent just upto calcium (just for lighter components).
- Newland balanced two components in a similar opening (for example Co and Ni), having various properties. For instance; Co and Ni with Fluorine, Chlorine, Bromine and Iodine.
- As per Newland, just 56 components existed in nature and no more components would be found in future.
Present endeavors for the order of components: Mendeleev’s Periodic Table, the Modern Periodic Table.
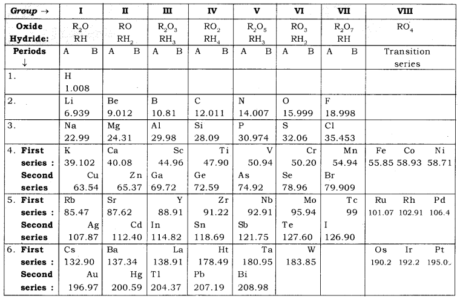
Mendeleev’s Periodic Table: Mendeleev’s periodic table depends on the physical and concoction properties of components and their atomic masses.
Mendeleev’s Periodic Law: According to this “The physical and concoction properties of the components are the periodic capacity of their atomic masses.”
Periodicity of Properties: The reiteration of properties of components after certain customary stretches is known as Periodicity of Properties.
Benefits of Mendeleev’s Periodic Table
- Mendeleev’s left empty spots in his table which gave a plan to the disclosure of new components. Model: Eka-boron, Eka-aluminum and Eka-silicon.
- Mendeleev’s periodic table was anticipated properties of a few unfamiliar components based on their situation in Mendeleev’s periodic table.
- It is valuable in remedying the far fetched atomic masses of certain components.
- could oblige in the Mendeleev’s periodic table without upsetting the periodic table after revelation.
Restrictions of Mendeleev’s Periodic Table
(a) No fixed situation for hydrogen: No right situation of the hydrogen iota was in Mendeleev’s periodic table.
Model: Position of hydrogen with soluble base metals and incandescent light (seventeenth gathering).
(b) No spot for isotopes: Position of isotopes were not chosen.
Model: Cl-35 and Cl-37.
(c) No ordinary pattern in atomic mass: Position of certain components with lower atomic masses before with higher atomic mass.
Model: Ni-58.7 before Co-58.9.
Mendeleev’s unique periodic table is duplicated in the table underneath
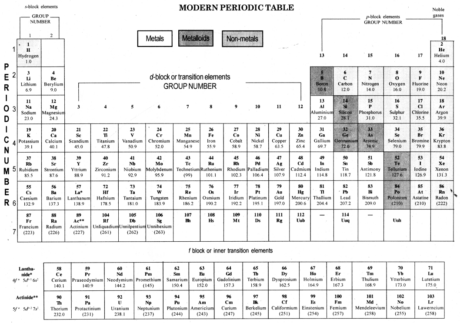
The Modern Periodic Table:In 1913, Henry Moseley indicated that the nuclear number of a component is a more basic property than its nuclear mass.
Modern Period Law: The physical and concoction properties of components are the periodic capacity of their nuclear number.
Modern periodic table depends on nuclear number of components.
Nuclear number (Z) is equivalent to the quantity of protons present in the core of a particle of a component.
Modern periodic table contains 18 vertical section known as gathering and seven even lines known as periods.
On moving from left to directly in a period, the quantity of valence electrons increments from 1 to 8 in the components present.
On moving from left to directly in a period, number of shell stays same.
All the components of a gathering of the periodic table have similar number of valence electrons.
Patterns in Modern Periodic Table: Valency, Atomic size, metallic and non-metallic characters, and Electronegativity.
(i) Valency: The valency of a component is dictated by the quantity of valence electrons present in the furthest shell of its particle (for example the joining limit of a component is known as its valency).
In Period: On moving from left to directly in a period, the valency first increments from 1 to 4 and afterward diminishes to zero (0).
![]()
In Groups: On moving through and through in a gathering, the valency stays same on the grounds that the quantity of valence electrons continues as before.
Model: Valency of first gathering components = 1 Valency of second gathering components = 2.
(ii) Atomic size: Atomic size alludes to range of a particle. It is a separation between the focal point of the core and the furthest shell of a detached molecule.
In Period : On moving from left to directly in a period, nuclear size reductions on the grounds that atomic charge increments.
Model: Size of second time span components: Li > Be > B > C > N > O > F
Highlight know: The nuclear size of respectable gases in comparing period is biggest
because of essence of completely filled electronic arrangement (for example complete octet).
In Group: Atomic size increments down the gathering in light of the fact that new shells are being
included dislike of the expansion in atomic charge.
Model ; Atomic size of first gathering component : Li < Na < K < Rb < Cs < Fr
Nuclear size of seventeenth gathering components : F < Cl < Br < I
(iii) Metallic character: It is the propensity of a particle to lose electrons. In Period: Along the period from left to right, metallic characters diminishes in light of the fact that an inclination to lose electron diminishes because of the expansion in atomic charge. Model: Metallic character of second time frame components: Li > Be > B > C >> N > O > F
In Group: Metallic character, while moving start to finish increments in light of the fact that the nuclear size and propensity to lose electrons increments.
Model: First gathering component : Li < Na < K < Rb < Cs
17th group elements: F < Cl < Br < I
(iv) Non-metallic character: It is propensity of a molecule to pick up electrons.
In Period: Along the period from left to right, non-metallic character increments since inclination to pick up electrons increments because of increment in core charge. Model ; Non-metallic character of second time frame components : Li < Be < B < C < N < O < F In Group: On moving through and through in a gathering, non-metallic character diminishes on the grounds that nuclear size increments and propensity to pick up electrons diminishes. Ex. Non-metallic character of seventeenth period component: F > Cl > Br > I
(v) Chemical Reactivity
In metals: Chemical reactivity of metals increments down the gathering since propensity to lose electrons increments. Model ; Li < Na < K < Rb < Cs (first gathering) In non-metals: Chemical reactivity of non-metals diminishes down the gathering since propensity to pick up electrons diminishes. Model: F > Cl > Br > I (seventeenth gathering)
(vi) Electronegativity: It is propensity of a component to draw in the mutual pair of electrons towards it in a covalently reinforced atom. It increments with increment of atomic charge or abatement in nuclear size.
Along the period electronegativity increments. Model ;Li < Be < B < C < N < O < F. Down the gathering electronegativity diminishes. Model ; Li > Na > K > Rb > Cs
F > Cl > Br > I
(vii) Nature of Oxides: Metal oxides are fundamental in nature. Ex. Na2O, MgO and so forth.
Non-metal oxides are acidic in nature. Ex. Cl2O7, SO3, P2O5,
On account of metal reactivity, it increments down the gathering due to the propensity to lose electrons increments.
On account of non-metal reactivity, diminishes down the gathering due to the inclination to pick up electrons diminishes.
Gathering: The vertical segments in Mendeleev’s, just as in Modern Periodic Table, are called gatherings.
Period: The level lines in the Modern Periodic Table and Mendeleev’s Periodic Table are called periods.
There are 18 gatherings and 7 (seven) periods in the Modern Periodic Table.
Nuclear size: The nuclear size might be pictured as the separation between the focal point of the core and the peripheral shell of a confined molecule.
The pattern of nuclear size (sweep) in descending a gathering: Ongoing down in a gathering of the Periodic Table, the nuclear size increments on the grounds that another shell of electrons is added to the iotas at each progression. There is an expansion in separation between the peripheral shell electrons and the core of the molecule.
The pattern of nuclear size (sweep) in moving from left to directly in a period: On moving from left to directly along a period, the size of iotas diminishes on the grounds that on moving from left to right, the nuclear number of components builds which implies that the quantity of protons and electrons in the particles increments. Because of the huge positive charge on the core, the electrons are pulled in more near the core and the size of the particle diminishes.
Qualities of sets of three of J.W. Dobereiner.
Components of a set of three show comparative concoction properties.
These components of a set of three show explicit patterns in their physical properties.
The nuclear mass of the center component was generally the normal of the nuclear masses of the other two components.
Model: Atomic mass of Na is 23 in the ternion Li, Na and K. This nuclear mass is the normal of the nuclear masses of Li and K which have nuclear masses 7 and 39 separately.
Triads as formed by Dobereiner.
1st Triad
Li – Lithium
Na – Sodium
K – Potassium
2nd Triad
Ca – Calcium
Sr – Strontium
Ba – Barium
3rd Triad
Cl – Chlorine
Br – Bromine
I – Iodine

