Hydrogen: Class 11 Chemistry NCERT Chapter 9

Key Features of NCERT Material for Class 11 Chemistry Chapter 9 – Hydrogen
In the last chapter 8, you learned about Redox Reactions. In this chapter: Hydrogen, you will learn about that did you realize that Hydrogen doesn’t have a place with any gathering or family in the Periodic Table? It has such exceptional properties that are not like some other element on our planet. Very nearly 66% of our Universe’s mass is made out of this exciting element! Let us find out about Hydrogen and a portion of its significant mixes.
We are on the whole mindful of our Modern Periodic table. Be that as it may, it took scientists years and numerous endeavors to show up at our present periodic table. What’s more, one central matter of dispute in the past endeavors was the situation of Hydrogen in the periodic table. Let us investigate the extraordinary situation of Hydrogen in the periodic table.
Location of Hydrogen in the Periodic Table
So in the event that you look at the periodic table, you will see Hydrogen is the primary element in the table. It is the littlest element on the table. It has nuclear number one, which implies it has just a single electron circling it its shell. Actually, Hydrogen has only one shell. It is likewise the lightest element on the periodic table.
Presently we realize that the situation of elements on the periodic table to a great extent relies upon their electronic configuration. Hydrogen has an automatic configuration of 1. It can dispose of one electron to achieve an honorable gas configuration. This trait of Hydrogen coordinates those of alkali metals. In any case, hydrogen iotas can likewise increase one electron like halogens. Let us perceive how this plays out.
Quick Revision notes:
- Electronic Configuration of Hydrogen 1s1
Position of Hydrogen in the periodic table: The location of Hydrogen in the periodic table isn’t defended because it takes after both alkali metals just as halogens.
- Resemblance of Hydrogen with Alkali Metals
(I) Electronic Configuration: Hydrogen contains one electron in its valence shell-like alkali metals.

(ii) Both hydrogen and alkali metals structure unipositive particles.
For instance,
Na — – > Na+ + e–
H — – > H+ + e–
(iii) Hydrogen and alkali metals the two show +1 oxidation state.
(iv) Hydrogen just as other alkali metals go about as decreasing operators.
(v) Both have an affinity for electronegative elements, For instance, Na2O, NaCl, H20, HCl.
- Resemblance with Halogens
(I) Electronic configuration: Hydrogen and halogen family both need one electron to satisfy the dormant gas configuration
(ii) Ionization vitality of Hydrogen is practically like halogens.
(iii) Hydrogen, just as halogens are Diatomic.
(iv) Many compounds of Hydrogen only as halogens are of covalent quality.
For instance, CH4, SiH4CCl4, SiCl4
- Occurrence of Hydrogen
Hydrogen is probably one of the most abundant element known to man. It is available in consolidated states as water, coal, creature, and vegetable issues. Every single natural compound contains Hydrogen as a fundamental constituent.
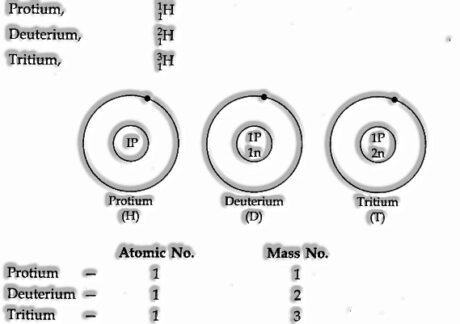
- Isotopes of Hydrogen
There are three isotopes of Hydrogen.
- Preparation of Dihydrogen, H2
- Preparation of Dihydrogen, H2
Lab Preparation of Dihydrogen
(i) It is prepared by the treatment of granulated zinc with dil HCl.
Zn + 2HCl ——–> ZnCl2 + H2
(ii) The action of zinc prepares it with some amount of aqueous alkali.
![]()
- Properties of Dihydrogen
(I) Dihydrogen is a vapid, unscented, and dull gas.
(ii) It is a burnable gas.
(iii) It is insoluble in water.
(iv) It is lighter than air.
Chemical properties:

Response with halogens: It responds with halogens, X2 to give hydrogen halides. HX.
- Hydrides
The hydrides are ordered into three kinds:
(I) Ionic or alkaline or salt-like hydrides
(ii) Covalent or atomic hydrides (iii) Metallic or non-stoichiometric hydrides.
- Ionic or Saline Hydrides
Hydrides are framed among Hydrogen and electropositive elements of gathering I and II having a place with s-square. These are known as stoichiometric compounds.
Properties of saline or ionic hydrides:
(I) The hydrides of lighter elements such as Li, Mg, and so on have substantial covalent characters.
(ii) Ionic hydrides are glasslike, non-unstable, and non-directing in a strong state.
(iii) They lead power in a liquid state and free Hydrogen at the anode.
- Covalent or Molecular Hydrides
These are parallel compounds of Hydrogen with non-metals having a place with p-square.
For instance, NH3, CH4, H20, HF are, for the most part, unstable compounds with low boiling focuses. They are delegated:
(I) Electron-Deficient Molecular Hydride: Molecular hydrides in which focal iota doesn’t have octet are called inadequate electron hydrides, e.g., BH3, MgH2, BeH2.
(ii) Electron exact hydrides: Those hydrides where the focal iota has its octet complete, e.g., bunch 14 hydrides. They are tetrahedral in the calculation.
(iii) Electron rich hydrides: Those particular metal hydrides that contain lone pairs of electrons are known as electron-rich more hydrides, e.g., NH3, PH3, H20, and H2S.
NH3 and PH3 have 1 lone pair, and H20 and H2S have two lone pairs of electrons.
- Metallic or Non-Stoichiometric Hydrides
These hydrides are otherwise called interstitial hydrides. Change metals bunch 3, 4, and 5 structure metallic hydrides. In bunch 6, chromium alone tends to shape CrH. Metals of 7, 8, and 9 don’t shape hydrides. This is called a hydride hole.
A most recent investigation shows that lone Ni, Pd, Ce, and Ac are interstitial in nature, which implies they can involve hydrogen iota in the interstitial sides. For the most part, the hydrides are non-stoichiometric, and their structure changes with temperature and weight, for instance, Ti H1.73, CeH2.7′, LaH2.8, and so forth.
These hydrides have metallic locks, and their properties are firmly identified with those of the parent metal. They are solid decreasing specialists in the vast majority of the cases because of the nearness of free hydrogen iota in the metal cross-section.
- Water
The human body has about 65%, and a few plants have almost 95% water.
Physical properties of water:
(I) The freezing purpose of water is 273.15 K and boiling point 373.15 K.
(ii) The maximum thickness of water at 4°C is 1 gm cm-3
(iii) It is a boring and dull fluid.
(iv) Due to hydrogen bonding with polar atoms, even covalent compounds like liquor and sugars break down in the water.
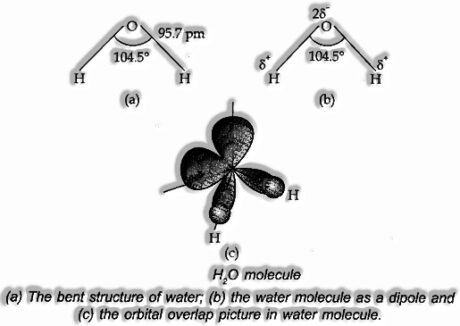
Structure of water: In the gas stage, it is a bowed atom with HOH bond edge 104.5° and O—H bond length of 95.7 pm. It is exceptionally polar. Its orbital cover picture is likewise demonstrated as follows.
Water in Crystalline Form:
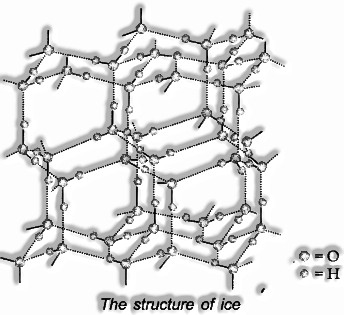
Ice is the crystalline type of water. At environmental pressure, ice takes shape in the hexagonal structure. At low temperatures, it gathers to the cubic structure. The density of ice is not as much as that of water. In this way, ice 3D shapes can skim on water.
Structure of ice:
Chemical Properties of Water:
(i) Amphoteric nature: It behaves like an amphoteric substance because it can act as an acid and a base.
Autoprotolysis of water also accounts for its amphoteric nature according to Bronsted-Lowry concept
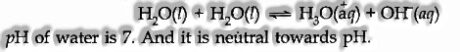
(ii) Oxidizing and Reducing Nature: Water can act as an oxidizing as well as a reducing agent.
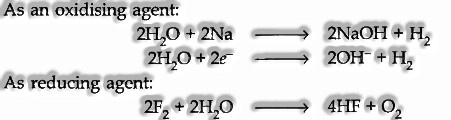
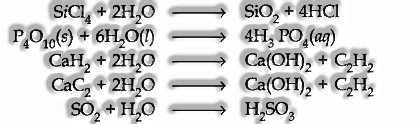
(iii) Hydrolysis Reaction: It has a dominant hydrating tendency. It can hydrolyze a large number of compounds such as oxides, halides, carbides, etc.
Hydrates Formation
From aqueous solutions, many salts can be crystallized as hydrated salts. Hydrates are of three types:
(i) Coordinated water
For example: [Ni(H20)6]2+ (N03–)2 and [Cr(H20)6]3+ 3CP
(ii) Interstitial water
For example, BaCl2. 2H20
(iii) Hydrogen bonded water
For example: [Cu(H20)4]2+ S042- H20 in CuS04.5H20
- Hard and Soft Water
Hard water: Water that doesn’t deliver lather with cleanser effectually is called hard water. The nearness of calcium and magnesium salts as hydrogen carbonate, chloride, and sulfate makes the water hard.
Sorts of Hardness of Water:
(I) Temporary hardness: It is because of the nearness of bicarbonates of calcium and magnesium in water. It is known as temporary since it tends to be effortlessly expelled by straightforward boiling of hard water.
(ii) Permanent hardness: It is because of the nearness of chlorides and sulfates of calcium and magnesium. It can’t be expelled on boiling water. Compound techniques can expel the permanent hardness of the water.
Soft water: Water which promptly frames lather with a cleanser is called delicate water.
For instance: downpour water, refined water.
- Hydrogen Peroxide (H202)
Preparation:
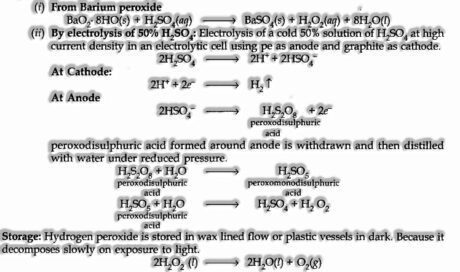
Employments of H202:
(I) It is utilized as a mellow disinfectant. It is showcased as perhydrol (an Antiseptic).
(ii) It is being used in the assembling of top-notch cleansers.
(iii) It is utilized in the blend of hydroquinone tartaric corrosive and certain food items and pharmaceuticals.
(iv) It is utilized as a blanching specialist for textiles, paper mash, and so on.
(v) It is utilized for contamination control treatment of local and mechanical effluents.
(vi) 93% of H202 is utilized as an oxidant for rocket fuel.
- Hard Water (D20)
It is utilized in the planning of other deuterium compounds.

Employments of D2O:
(I) It is utilized as a mediator in atomic reactors.
(ii) It is utilized in the trade reaction investigation of reaction systems.
- Hydrogen as a Fuel
Hydrogen Economy: The fundamental rule of the hydrogen economy is the transportation and capacity of vitality as fluid or gaseous dihydrogen. Bit of leeway is that vitality is sent as dihydrogen and not as an electric force.
Preferred position as a fuel:
– It is utilized as energy units for the age of electric force.
– One significant preferred position of ignition of Hydrogen is that it delivers almost no contamination, and there isn’t any outflow of unburnt carbon particles as smoke.
– It is evident from the investigation that dihydrogen in the gaseous state, just as in condensed structure, delivers more vitality on burning compared with the other fuel customarily utilized.
– 5% of dihydrogen is blended in CNG for use in four-wheeler vehicles.
Questions
Q: Hydrogen is chiefly found in the consolidated state like in water and not the free state. Valid or False?
Ans: This announcement is True. The world’s hull contains almost 1% of Hydrogen by weight. In a free state, Hydrogen happens just as follows in the environment. It is mostly seen in the United state of America and not the open state. Its fundamental sources are water, acids, fundamental issues, and so forth.

