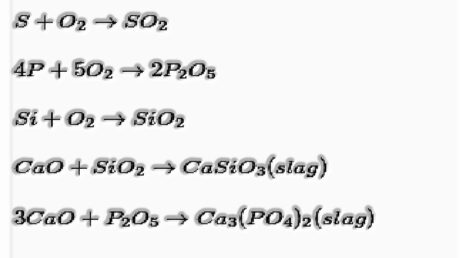General Principles And Processes Of Isolation Of Elements: Class 12 Chemistry NCERT Chapter 6

NCERT Study Material for Class 12 Chemistry Chapter 6 – General Principles And Processes Of Isolation Of Elements
In the last chapter 5, you learned about Surface Chemistry. In this chapter you will learn about General Principles And Processes Of Isolation Of Elements in detail.
There are around 91 metals known to man. Be that as it may, from these solitary Silver, Gold, Copper, and Platinum occur in enormous amounts in their free state. To get other unadulterated metals, we use principles of chemistry, specifically extraction and isolation. Let us further study the general principles and processes of isolation of elements.
Metals are especially useful substances. It is all around acknowledged that our innovatively advanced lives would not be possible without metals. Their physical properties such as electric conductivity, density, flexibility, and so forth make them ideal for industrial use. Presently let us investigate the extraction of metals from their ores.
Quick revision notes
Minerals: The commonly occurring compound substances in the world’s crust that are gotten by mining are known as minerals.
Metals could possibly be separated productively from them.
Ores: The rough materials which contain a sufficient quantity of mineral so that the metal can be separated gainfully or economically are known as ores.
Gangue: The hearty or undesirable materials present in the ore is known as gangue.
Metallurgy: The entire scientific and innovative process used to isolate the metal from its ores is metallurgy.
Boss Ores and Methods of Extraction of Some Common Metals:
Sodium
Found as: Rock salt (NaCl), Feldspar ()
Removal method: Electrolysis of fused NaCl or NaCl/
Derivation: Sodium is exceptionally reactive, and thus, it reacts with water.
Copper
![]()
- Found as: Copper pyrites ()(), Malachite (), Cuprite () Copper glance ()
- Removal method: Roasting of sulphide partially and reduction.
- Derivation: It is self-reduction in a specially designed converter. Sulphuric acid leaching is also utilized.
Aluminum
- Found as: Bauxite:( where 0<x<1), Cryolite (),Kaolinite ()
- Removal method: Electrolysis of Al2O3 dissolved in liquid cryolite or Na3AlCl6
- Derivation: In the extraction of Al, A decent source of power is required
Zinc
- Found as: Sphalerite(ZnS), Zincite (ZnO), Calamine ()
- Removal method: Roasting and afterward, reduction with carbon.
- Derivation: The metal might be filtered by fractional distillation.
Lead
- Found as: Galena (PbS)
- Removal method: Roasting of the sulfide ore and afterward reduction of the oxide.
- Derivation: Sulphide ore is concentrated by the froth floatation process.
Silver
- Found as: Argentite ()
- Removal method: Sodium cyanide leaching of the sulphide ore and finally replacement of Ag by Zn.
- Inference: It involves complex formation and displacement.
Gold
- Found as: Native, small amounts in numerous ores such as those of copper and silver
- Removal method: Cyanide leaching, same as in the case of silver 3.
- Surmising: Gold reacts with cyanide to frame complex
Iron
- Found as: Haematite (), Magnetite (), Siderite (),Iron pyrites ()
- Removal method: Reduction with the assistance of CO and coke in a blast heater.
- Surmising: Limestone is included as motion, which removes as calcium silicate (slag) floats over liquid iron and prevents its oxidation. Temperatures moving toward 2170 K is required.
- Steps of metallurgy:
- Concentration of ore
- Conversion of concentrated ore to oxide.
- Reduction of oxide to metal
- Refining of metal
- The Concentration of ore: The process of expulsion of unwanted materials like sand, dirt, rocks, and so on from the ore is known as concentration, ore – dressing, or benefaction. It involves several steps which rely on physical properties of metal compound and pollution (gangue). The sort of metal, accessible facilities, and environmental factors are also thought about.
- Hydraulic washing (or gravity separation):This technique is based on the contrast in ore and gangue particles’ densities. Ore is washed with water under tension so that lighter impurities are washed away, whereas weighty ores are deserted.
- Magnetic separation: This strategy is based on the distinction in magnetic and non – magnetic properties of two ore (unadulterated and sullied). This strategy is used to eliminate the ore particles of tungsten from cassiterite ().and also employed to concentrate magnetite (), chromite () and pyrolusite () from an undesirable gangue.
- Foam floatation process: It is based on the rule that sulfide ores are preferentially wetted by the pine oil or unsaturated fats or xanthates.Collectors are added to upgrade the non-wettability of the mineral particles. Froth stabilizers such as cresols, aniline and so forth., are added to stabilize the foam. On the off chance that two sulfide ores are present, it is possible to separate the two sulfide ores by adjusting the oil proportion to water or by including depressants. For instance, on account of ore with ZnS and PbS in it, the depressant that is used is NaCN. It provides resistance to ZnS and selectively prevents ZnS from coming to froth. However, it allows PbS to go with the foam.
- Leaching (Chemical separation):It is a process where ore is treated with a suitable solvent that dissolves the ore yet not the impurities.
- Purification of Bauxite by leaching ( Baeyer’s process):

- a) First step:
- b) Second step:
- c) Third step:
- d) The Concentration of Silver and Gold Ores by Leaching:
Formation of oxide from the ore: This is quite easy to lessen oxide than sulfide ore carbonate. Hence, the oxide should be made out of ore by any of the accompanying technique:
- Roasting
- calcination
- Roasting:
- It is a process where ore is warmed in a regular supply of air at a temperature underneath the metal’s melting purpose for the oxide to come out of the given ore.
- The ores of Sulphide are transformed into oxide by roasting.
- It is employed to eliminate impurities as volatile oxides also.
- For instance –

- Calcination
- It is a heating ore process in restricted air supply to make oxides out of carbonate ore.
- The carbonate ores are transformed into oxides by roasting.
- It is also used to eliminate moisture and volatile impurities. .
- For instance –

- Reduction of oxide to metal: The technique of transforming metal oxide into metal is called reduction. It needs a suitable diminishing operator relying on the reactivity or lessening the intensity of metal. The common decreasing agents used are carbon or carbon monoxide or some other metals like Al, Mg, etc.
- Thermodynamic principles of metallurgy: Some basic thermodynamics concepts help understand the conditions of temperature and select suitable lessening operator in metallurgical processes:
- At any temperature, Gibbs free energy change is denoted by ΔG = ΔH – TΔS where ΔG is free energy change, ΔH is enthalpy change and ΔS is the change in entropy.


- Types of iron:
- Pig iron: The iron acquired from blast heaters is called pig iron. It is a sullied form of iron containing 4% carbon and a small measure of S,.P, Si, and Mn. It very well may be cast into an assortment of shapes.
- Cast iron: It is formed by melting pig iron with scrap iron and coke using hot air blast. It contains about 3% carbon content. It is incredibly hard and weak.
- Wrought iron: It is the purest type of commercial iron. It is also called flexible iron. It is set up by oxidative refining of pig iron in a reverberatory heater fixed with haematite, which oxidizes carbon to carbon monoxide.

The substance which reacts with debasement to shape slag is called transition; for example, limestone is motion.
The metal is eliminated and liberated from slag.
- Electrolytic Reduction (Hall – Heroult Process): The unadulterated bauxite ore is blended with cryolite () or lowers its melting point and increases electrical conductivity. The liquid blend is electrolyzed using various graphite rods as anode and carbon lining as a cathode.The graphite anode reduces metal oxide to metal.

- Electrolysis of molten NaCl:
At cathode:
At anode:
Thus sodium metal is gotten at the cathode, and (g) is freed at the anode.
-
- Refining: It is the process of converting a polluted metal into unadulterated metal contingent on the idea of metal.
- Distillation: It is the process used to clean those metals which have low breaking points, e.g., zinc, mercury, sodium, potassium. Polluted metal is warmed to convert it into vapors, which changes into the pure metal on condensation and is obtained as distillate.
- Liquation: This technique can sanitize those metals with impurities whose melting points are higher than metal. In this technique, Sn metal can be filtered. Tin containing iron as impurities warmed on the head of the sloping heater. Tin after melting flows down the sloping surface where iron is deserted and unadulterated tin is gotten.
- Electrolytic refining:
- In this technique, unclean metal is taken as an anode.
- Unadulterated metal is taken as a cathode.
- A salt of metal is used as the electrolyte.
At the point when an electric current is passed, sullied metal forms metal ions which are discharged at cathode shaping unadulterated metal.
At anode:
At cathode:
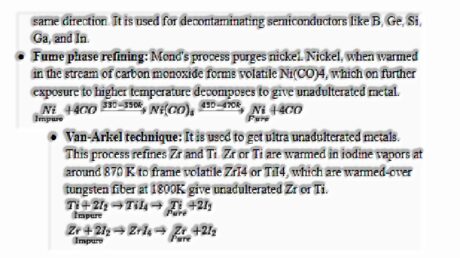
- Zone refining: It is based on the rule that impurities are more soluble in the soften than in the solid-state metal. The polluted metal is warmed with circular heaters’ assistance toward one side of the toxic metal bar. The liquid zone moves forward alongside the warmer with impurities and reaches the opposite end and is discarded. Unadulterated metal crystallizes out of the liquefy. The process is rehashed several times, and warmer is moved in the same direction. It is used for decontaminating semiconductors like B, Ge, Si, Ga, and In.

- Fume phase refining: Mond’s process purges nickel. Nickel, when warmed in the stream of carbon monoxide forms volatile Ni(CO)4, which on further exposure to higher temperature decomposes to give unadulterated metal.
- Van-Arkel technique: It is used to get ultra unadulterated metals. This process refines Zr and Ti. Zr or Ti are warmed in iodine vapors at around 870 K to frame volatile ZrI4 or TiI4, which are warmed-over tungsten fiber at 1800K give unadulterated Zr or Ti.
Chromatographic method: It is based on the rule of separation or purification by chromatography based on differential adsorption on an adsorbent. In column chromatography, it is used as adsorbent. The blend to be separated is taken in a suitable solvent and applied to the column. They are then eluted out with suitable solvent (eluent). The feebly adsorbed component is eluted first. This strategy is suitable for such elements that are accessible just in minute quantities. The impurities are not a lot of variety in their compound conduct from the component to be purged.
Question:
Q: In the extraction of copper from its sulfide ore, the metal forms by reduction of Cu2O with which of the accompanying?
- ZnS
- CO
- Cu2S
- None of the above mentioned
Ans: The correct option is “C.” Compounds of specific metals lessen to metals without using any additional diminishing operator ores of Cu, Pb, Hg, etc. Their sulfide ores are partially roasted to give some oxide. This oxide is presently diminished to the metal by the rest of the sulfide ore at raised temperatures in the absence of air. This process is the thing that we call self-reduction.
Cu2S + 2Cu2O →6Cu + SO2
(General Principles And Processes Of Isolation Of Elements: Class 12)
.