Changes Around us: Class 6 Science NCERT Chapter 6

Key Features of NCERT Material for Class 6 Science Chapter 6 – Changes Around Us
In the last chapter 0f NCERT class 6 science: Separation of Substances you studied different methods of separation and to remove impurities. You also studied a few examples of these techniques. In this chapter: changes around us, you will learn the different types of changes that take place in our surrounding. You will look at a few examples of these changes.
Quick revision notes
We can accomplish a change in a substance by doing any one of the below techniques:
- Heating.
- Applying force.
- Mixing it in with something distinctive.
Changes achieved by heating: Heating of an article impacts in one or various likely ways.
- A few articles get hot yet don’t change in some other way.
- A couple of things get hot and moreover increase in size.
- A few things get hot and begin to bum.
- A couple of things get hot and change their state.
Changes by applying pressure: When we apply power to a thing,
- We can change its shape and size.
- Air can be compacted.
- Metals can be beaten into thin sheets.
- Flexibility can be expanded.
- Cotton can be spun into thin strings.
Changes by mixing a substance in with another: We can understand a change in a substance by blending it in with another. For example, making an arrangement by blending water dissolvable substances in water.
Metals create warming and concession to cooling.
Concoction changes:
These are where synthetic properties of a substance change, and which moulds another substance. For example, making food.
Physical changes:
These are the changes wherein simply a physical property of a substance changes and there is no formation of a new substance.
Characteristics of physical changes:
- No formation of new substances.
- Products are the same as the reactants.
- These changes are reversible.
Characteristics of substance changes:
- Properties of things are not exactly equal to the properties of reactants.
- Most by far of the substance changes are irreversible.
- The changes mostly aren’t reversible
Reversible changes:
These are the changes one can reverse it. For example, the flexibility of elastic.
Irreversible changes:
These are the changes which can’t be reclaimed to its original state. For example, using paper.
Melting point:
A consistent temperature at which solid starts softening. This temperature is known as the melting point for that solid.
Freezing:
A strategy wherein liquid changes into the solid structure is freezing.
Force:
A push or a draw following up on a body which will, by and large, change its condition of rest or development is a force.
Common changes:
The changes which occur in nature in solitude are called common changes. For example, change of day to night and vice versa, change of seasons every year.
Slow changes:
The changes which put aside longer exertion to happen are slow changes. For example, rusting of the iron, decaying of tooth.
Changes:
Many changes happen around all of only us, e.g., blooming of sprout and a short time later it is shrunk. We can in this manner bring a change, e.g., change in the size of an inflatable by blowing air in it.
Pressure:
A strategy wherein a thing diminishes is pressure.
Evaporation:
A strategy wherein liquid changes into gas is evaporation.
Extension:
A method wherein an article increments in size, e.g., metals create warming.
Melting:
A method where solid melts to go into a liquid on warming is melting.
Reversible And Irreversible Changes:
Changes that occur around us can be exhaustively arranged as reversible or irreversible depending upon whether they can be convoluted.
Reversible Changes:
Changes that can be turned back are called reversible changes.
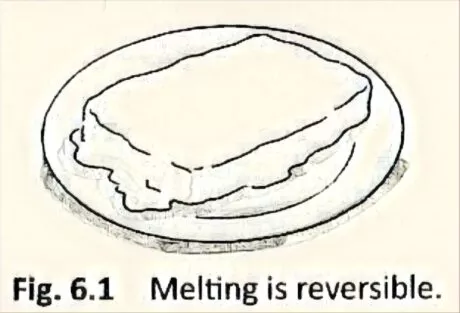
What happens a solidified yoghurt if you don’t cover it? It melts. OK have the option to change the fluid yoghurt again into a solid? To be sure! Essentially keep it in the cooler. You can change liquid yoghurt back to its solid structure. Appropriately, melting is a reversible change. The melting of spread and chocolate are also reversible changes (Fig. 6.1).
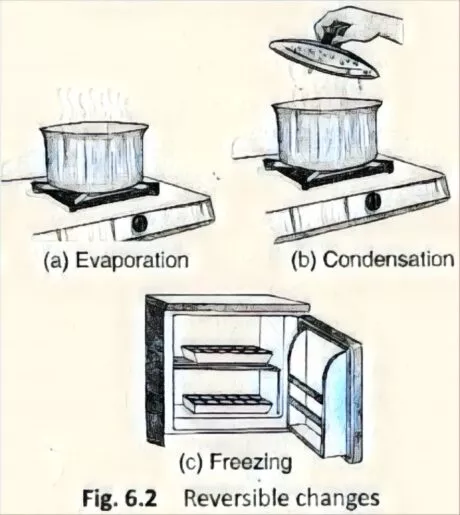
Something should be said about changes like condensation, freezing, and evaporation of materials. In case you take out some ice cubes from the cooler and keep them outside, the ice cubes will acclimatize heat from the encompassing and melting. Exactly when this water (fluid ice) is warmed for a long time, it starts converting to vapour (liquid starts to evaporate) and steam escapes from the holder [Fig. 6.2(a)]. Directly, in case you hold a top over the compartment, the steam will again liquify or assemble into little dabs of water on collaborating with the cold spread [Fig. 6.2(b)]. This water can be chilled off further and a short time later kept in the cooler to outline ice again [Fig. 6.2(c)]. Thusly, the three physical conditions of the water are reversible and warming and cooling can change them.
Irreversible Changes:
Changes that can’t be turned are called irreversible changes.
There are incalculable irreversible changes that happen around us. These result in another material being made, which could possibly be important. A couple of cases of irreversible changes are below.
- The maturing of natural items is an irreversible change since it is crazy to hope to get back the unrefined characteristic items from matured or create ones.
- Growing of blossoms is an irreversible change since flowers can’t change over into buds.
- Milk is destroyed if you do not refrigerate it, particularly in summer. Its alluded to coagulating or souring of milk and is an irreversible change. One can coagulate milk by adding lemon juice to deplete for making curds or paneer.

- Burning paper is an irreversible change. After burning paper results in ash residue. This new substance contrasts from the paper in its appearance and properties.
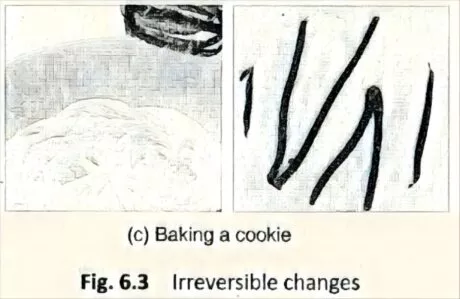
- Preparing food is an irreversible change since we can’t get back the raw food. For example, after a cooking a cake using flour, egg, milk, chocolate, etc., we can’t get back the ingredients(Fig. 6.3).

- Burning of the candle is consistently referred to for an example of physical change since what we see rapidly is liquefying of wax that sets on cooling. Regardless, when a fire burns, the wax is encountering two changes all the while: first it liquefies, and a short time later it burns. Actually, melted wax burns. The wax burns on the wick – the wick isn’t burning, it is just the wax on it.
Physical And Chemical Changes:
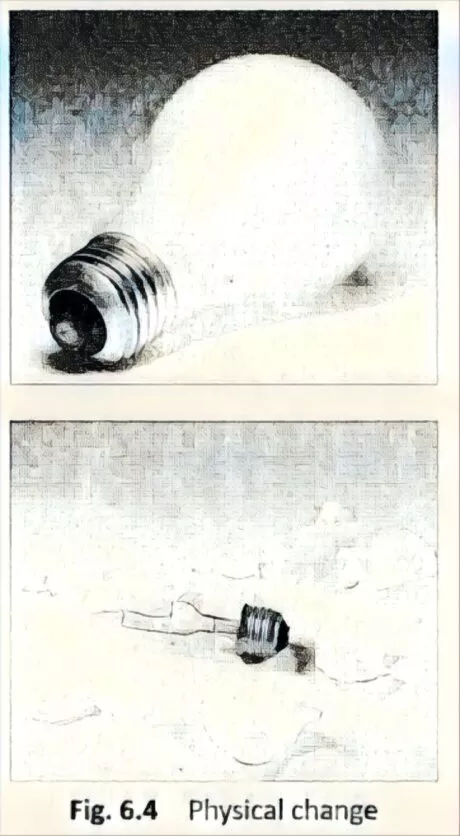
Changes which does not lead to the production of new substances are called physical changes. For example, breaking of glass (Fig. 6.4), freezing of water, paper being torn, etc.
Changes which leads to the formation of new substances with new and different properties are chemical changes. Preparing food, burning of substances are concoction changes as totally new substances are moulded. Burning of light wax releases carbon dioxide and water fumes (new substances).
Expansion And Contraction Of Materials:
A couple of materials create a warming and some agreement on cooling. Warming makes the particles (that structure the material) stretch out or end up being free. Cooling makes the particles (that structure the material) contract or become tight.
The proportion of extension shifts in solids, liquids, Fig-6-4 Physical change additionally, gases. Gases expand the most while solids develop the least.
Table 6.1 gives a couple of examples of development.

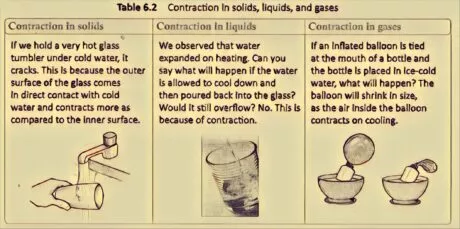
Cooling is opposite for warming. Cooling settles on a material. The contraction in Solid is the least and the most in gases. Table 6.2 records a couple of cases of constriction.
Utilizations of Expansion and Contraction:
We can use expansion by warming in a couple of ordinary activities.
Heating can open the metal front of a jam holder. The compartment is vexed and basically the top is dunked in bubbling water. After some time, one can open the top adequately as the spread gets broad.
The way that materials create warming is used in thermometers. In various thermometers, mercury is used. Exactly when the bulb of the thermometer communicates with a hot thing, the mercury expands and its level climbs in the glass tube, indicating the temperature.
Why the electric lines are not hung firmly between the posts? Wires in the outside condition are presented to atmosphere conditions stretching out from exceptional hot to cold temperatures. A tight wire on compression in winters can snap.
Reversible change:
A change that can be turned around is a reversible change.
Irreversible change:
A change that can’t be turned around is an irreversible change.
Physical change:
A change where there is no formation of new substances is a physical change.
Concoction change:
A change which leads to the formation of new substances with different properties is a synthetic change.
Changes happen around us and moreover inside us.
A couple of changes are reversible, while some others are irreversible.
A couple of changes are physical changes; some are concoction changes.
Warming causes development in a material.
Cooling causes constriction in a material.
Gases expand the most and solids develop the least.